| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website http://www.theijcp.org |
Original Article
Volume 1, Number 2-3, June 2012, pages 55-62
Febrile Seizures With Pandemic (H1N1) 2009 Virus Infection
Tatsuo Fuchigamia, b, Emiko Momokia, Maki Hasegawaa, Yuki Imaia, Ayako Nakamuraa, Katsuya Saitoa, Teruaki Ishikawaa, Chikako Arakawaa, Koji Hashimotoa, Yukihiko Fujitaa, Yasuji Inamoa, Hideo Mugishimaa
aDepartment of Pediatrics and Child Health, Nihon University School of Medicine, Tokyo, Japan
bCorresponding author: Tatsuo Fuchigami. Department of Pediatrics and Child Health, Nihon University School of Medicine, 30-1 Oyaguchi-Kamicho, Itabashi-ku, Tokyo 173-8610, Japan
Manuscript accepted for publication June 11, 2012
Short title: FSs With Pandemic (H1N1) 2009 Virus Infection
doi: https://doi.org/10.4021/ijcp15w
| Abstract | ▴Top |
Background: In April 2009, a novel influenza A (H1N1) pdm virus was identified in Mexico, and spread quickly around the world.
Methods: We treated 90 patients aged 0 - 14 years with influenza-associated febrile seizures due to this virus, who presented at our hospital between July 2009 and March 2010. We investigated the clinical characteristics of the patients. We also compared the clinical presentation of children with febrile seizures due to pandemic A (H1N1), seasonal influenza, and non-influenza infection.
Results: The average age of patients with febrile seizures and influenza A (H1N1) pdm was 4.3 years, which was significantly higher than that for seasonal influenza, 3.1 years, and non-influenza infection, 2.1 years.
Conclusions: There was no significant difference in neurological symptoms between the patients with febrile seizures due to seasonal influenza and 2009 pandemic influenza. However, febrile seizures with influenza A (H1N1) pdm occurred in older children compared with seasonal influenza and non-influenza infection.
Keywords: Febrile seizures; Influenza; Pandemic influenza; Seasonal influenza; Influenza A(H1N1) pdm virus; Children; Neurological symptoms; Japan
| Introduction | ▴Top |
Febrile seizures (FSs) are seizures (most commonly convulsive, but occasionally non-convulsive) that occur in infants and young children, in association with a fever of 38.0 °C or higher but without evidence of any definite causative disease (abnormality), such as a central nervous system infection, metabolic abnormality or intoxication [1]. The prevalence of FSs is relatively high (7-8%) in Japan [1]. In the United States and Europe, the incidence of FSs is 2-5% [2] for the entire pediatric population. We have already reported the clinical characteristics of FSs [3, 4], and its association with influenza virus infection has also been reported [3, 5-10].
In the spring of 2009, a novel influenza A (H1N1) pdm virus was identified in Mexico, the United States and Canada [11], and spread quickly around the world. The first patient in Japan was identified during May 2009. A few reports on FSs associated with 2009 pandemic influenza have been published [12–14]. However, the clinical features of FSs associated with 2009 pandemic influenza have not yet been reported. We treated 90 patients with influenza-associated FSs due to this virus, and investigated the clinical characteristics. To clarify the features of FSs associated with 2009 pandemic influenza, we also compared clinical characteristics of FSs in historical patients with seasonal influenza and those with 2009 pandemic influenza.
| Materials and Methods | ▴Top |
We studied patients with FSs due to influenza A (H1N1) pdm virus, who presented at the Department of General Pediatrics, Nihon University Nerima-Hikarigaoka Hospital, Tokyo, Japan between July 2009 and March 2010. They were diagnosed with FSs according to the clinical practice guideline on the neurodiagnostic evaluation of children with simple febrile seizures [2]. Seizure events in infants or young children accompanied by a temperature of 38.0 °C or higher were defined as FSs. Patients with seizures with definable causes such as acute central nervous system infections, acute encephalopathy, intracranial lesions, and acute abnormal metabolism, as well as seizures associated with fever in patients with a history of afebrile seizures were excluded from the FS group. Influenza infection tends to cause FSs in older children [10], therefore, no upper age limit was set in our study, although patients aged < 6 months were excluded. The diagnosis of influenza was established by rapid antigen-detection assay from nasopharyngeal swabs. We used the rapid influenza diagnostic test (QuickNavi™ - Flu; Denka Seiken Co. Ltd., Tokyo, Japan) for the diagnosis of influenza infection. This rapid test was designed for the diagnosis of seasonal influenza infection and to distinguish between influenza A and B. Nasopharyngeal swabs were sent to the laboratory for microbiological identification. However, pandemic (H1N1) 2009 virus was not determined by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) for all patients with influenza associated FS. According to the Infectious Agents Surveillance Report in Japan [15], nearly 100% of the influenza viruses detected/isolated from influenza cases during the same period were influenza A (H1N1) pdm. We considered that influenza in our patients was caused by pandemic (H1N1) 2009 virus. The clinical characteristics were evaluated.
To clarify the features of FSs with 2009 pandemic influenza, we also compared clinical characteristics of FSs in historical patients with seasonal influenza or 2009 pandemic influenza. Ninety-five patients (60 male, 35 female) had FSs associated with seasonal influenza. The diagnosis of influenza was established by rapid antigen-detection assay from nasopharyngeal swabs from all patients. We used the rapid influenza diagnostic test (Quick S-Influ A/B “Seiken”; Denka Seiken Co. Ltd.) for the diagnosis of influenza infection. Ninety-five patients with seasonal influenza (44 influenza A, 36 influenza B, 15 no defined type) were treated at our hospital between November 2004 and April 2005 (the 2004/2005 influenza season) [3]. The 15 patients with no defined type of seasonal influenza were excluded from the study. Thus, 80 patients with FSs associated with seasonal influenza (54 male, 26 female) were enrolled in the present study. Two hundred and fifty-two patients with non-influenza associated FSs (154 male, 98 female) treated at our hospital during the same seasonal influenza season were also studied.
Statistical analysis was performed using SPSS 10.0 for Windows. Univariate analysis was performed to identify differences between patient groups with and without each special item, using the t test, χ2 test, and Mann-Whitney non-parametric test, as appropriate. Differences were considered statistically significant at P < 0.05.
| Results | ▴Top |
Children with 2009 H1N1 influenza
There were 1768 (975 male, 793 female) children with influenza, aged 0 - 15 years, in our hospital between July 2009 and March 2010 (Fig. 1). Two hundred and twenty-five patients (12.7%) were 0 - 3 years old, 437 (24.7%) were 4 - 6 years old, 841 (47.6%) were 7 - 12 years old, and 265 (15.0%) were > 13 years old. The peak period for the patients with 2009 H1N1 influenza was from October 2009 to November 2009 (Fig. 2). The diagnosis of influenza was established by rapid antigen-detection assay from nasopharyngeal swabs from all patients. However, pandemic (H1N1) 2009 virus was not determined by real-time RT-PCR for all patients with influenza. According to the Infectious Agents Surveillance Report in Japan [15], nearly 100% of the influenza viruses detected/isolated from influenza cases during the same period were A (H1N1) pdm. We considered that the influenza infection in our patients was caused by pandemic (H1N1) 2009 virus.
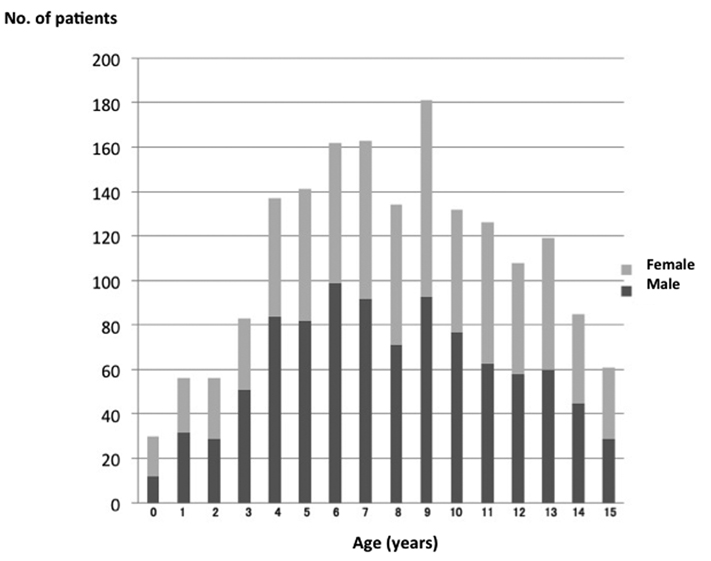 Click for large image | Figure 1. Age distribution of children with influenza A (H1N1) pdm infection. |
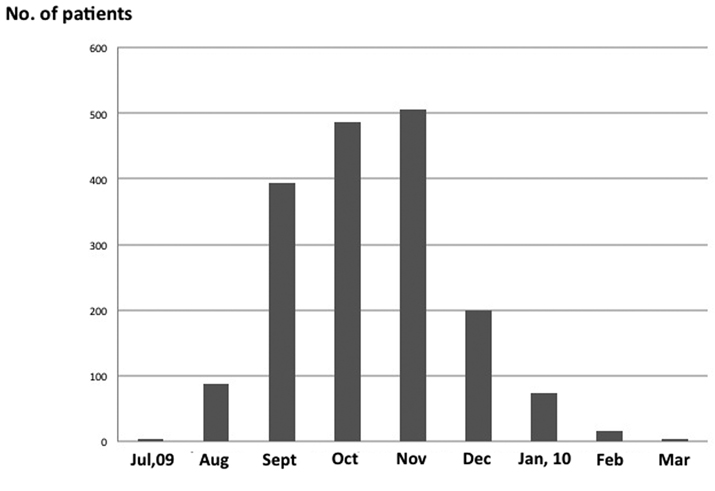 Click for large image | Figure 2. Seasonal distribution of children with influenza A (H1N1) pdm infection. |
Characteristics of FSs in children with 2009 H1N1 influenza (Table 1)
Ninety (5.1%) of 1768 children diagnosed with novel influenza A (H1N1) infection developed FSs during their course of illness. Ninety patients (60 male and 30 female; age 0 - 14 years, average, 4.3 years) had influenza FSs (Fig. 3). There were 23 patients (25.6%) with FSs aged ≥ 6 years. Among the 90 patients, 78 were outpatients (86.7%) and 12 were inpatients (13.3%). Body temperature at seizure occurrence was 39.3 ± 0.9 °C. Twenty-five patients (27.8%) showed a positive history of seizures in first-degree relatives. The time from fever to seizure occurrence was within 24 hours in 83 patients (92.2%), and after 24 hours in 7 (7.8%). Forty-one patients (45.6%) had no previous FSs. Seizure manifestation showed the simple type in 82 patients (91.1%) and complex type in 8 (8.9%). The average seizure duration in the 90 children was 4.8 ± 8.0 minutes. The duration was < 5 minutes in 68 patients (75.6%), and the seizures persisted for 15 minutes or longer in 6 patients (6.6%). Fifty-one children did not take medication, and 31 took medication: 18 oseltamivir, 6 antihistamine, and 4 antipyretics.
 Click to view | Table 1. Characteristics of FSs in children with 2009 H1N1 influenza infection. |
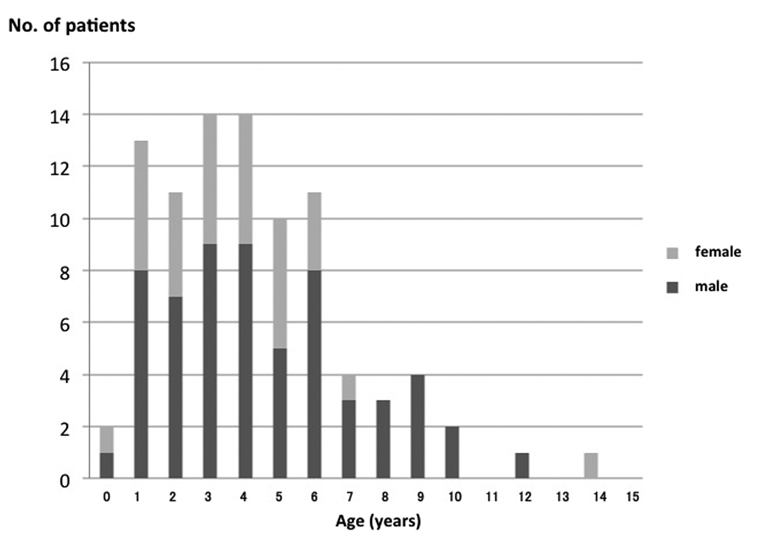 Click for large image | Figure 3. Age distribution of FSs in children with influenza A (H1N1) pdm infection. |
Comparison of children with FSs and influenza A (H1N1) pdm, seasonal influenza, and non-influenza infection (Table 2)
The male-to-female ratio and body temperature at seizure occurrence did not differ among the children with FSs and influenza A (H1N1) pdm, seasonal influenza, and non-influenza infection.
 Click to view | Table 2. Comparison of children with FSs in influenza A (H1N1) pdm, seasonal influenza, and non-influenza infection. |
The mean age of the patients with pandemic influenza was 4.3 years, which was significantly higher than in patients with seasonal influenza (3.1 years; P < 0.05), and non-influenza infection (2.1 years; P < 0.01). The proportion of patients aged ≥ 6 years with FSs and influenza A (H1N1) pdm infection was 25.6%, which was also significantly higher than in seasonal influenza (11.3%; P < 0.05) and non-influenza infection (4.3%; P < 0.01). The proportion of patients with a positive history of seizures in first-degree relatives did not differ among the children with FSs and influenza A (H1N1) pdm, seasonal influenza, and non-influenza infection. Among patients with seasonal influenza, the proportion with seizure occurrence within 24 hours from fever onset was 70%, which was significantly lower than in influenza A (H1N1) pdm (92.2%; P < 0.01) and non-influenza infection (88.5%; P < 0.01). Among patients with influenza A (H1N1) pdm infection, 45.6% had no previous history of FSs, which was significantly lower than in those with non-influenza infection (61.1%; P < 0.01). The proportion of patients with simple type seizure manifestation did not differ among children with influenza A (H1N1) pdm, seasonal influenza, and non-influenza infection. Among patient with influenza A (H1N1) pdm infection, 75.6% had seizure duration < 5 minutes, which was significantly lower than in those with seasonal influenza (87.5%; P < 0.05) and non-influenza infection (90.1%; P < 0.01).
Among hospitalized children with influenza A (H1N1) pdm infection, 13.3% were hospitalized, which was not significantly higher than among those with seasonal influenza (8.8%) and non-influenza infection (7.1%). The mean age of the hospitalized children with pandemic influenza was 4.5 years, which was not significantly higher than those with seasonal influenza (2.9 years) and non-influenza infection (2.1 years).
| Discussion | ▴Top |
The US Centers for Disease Control and Prevention first identified two cases of novel swine-origin influenza (H1N1) infection in April 2009, and outbreaks of this infection were subsequently identified worldwide. Given that most cases of the illness occur in young people, there is great concern regarding the incidence and mortality of serious complications, such as encephalopathy, among children.
On November 13, 2009, the Japan Pediatric Society reported surveillance data concerning 60 deaths associated with pandemic (H1N1) 2009 virus in children [16]. The main causes of death were sudden death and rapidly progressive severe pneumonia. We have also described the clinical aspects of pandemic (H1N1) 2009 virus infection in children who develop pneumonia [17], plastic bronchitis [18] or spontaneous pneumomediastinum [19], and encephalopathy [20]. However, only a few reports on FSs associated with 2009 pandemic influenza have been published [12-14]. In the present study, 5.1% of children diagnosed with novel influenza A (H1N1) infection developed FSs during their course of illness. However, in the previous study, 21.1% of children diagnosed with seasonal influenza infection developed FSs [3]. The proportion of patients with pandemic (H1N1) 2009 virus infection who developed FSs was 5.1%, which was significantly lower than in those with seasonal influenza (21.1%; P < 0.01). In Dallas, TX, United States, during April 22 - July 20, 2009, among 405 persons with laboratory-confirmed novel influenza A (H1N1) virus infection, four patients aged 7 - 17 years had associated neurological complications [12]. Three of these with acute neurological complications were diagnosed with encephalopathy. Only one patient aged 7 years had FS. These results indicated the incidence of FSs caused by novel A (H1N1) virus infection was low.
To clarify the features of FSs in patients with 2009 pandemic influenza, we compared the clinical features of FSs in historical patients with those in patients with seasonal influenza and 2009 pandemic influenza.
Generally, most FSs occur between 6 months and 3 years of age, with a peak incidence at 18 months [21, 22]. Approximately 6-15% occur after 4 years, and onset after 6 years is unusual [23]. However, the mean age of patients with FSs and pandemic influenza was 4.3 years, and 25.6% of patients with FSs and influenza A (H1N1) pdm infection were ≥ 6 years old, which were significantly higher than in patients with seasonal influenza and non-influenza infection. In this study, the oldest FS patient was a 14-year-old girl with influenza A (H1N1) pdm. Tan et al. [13] have reported a 16-year-old woman with influenza A (H1N1) pdm and first-onset seizure. These results showed that, in patients with influenza A (H1N1) pdm, FSs occurred in older children compared with seasonal influenza and non-influenza infection. Ostovar et al. have compared the clinical features and disease course of seasonal and pandemic influenza in hospitalized children in the Unites States [24]. They found that the median age of patients with pandemic A (H1N1) influenza was 6.5 years, which was significantly higher than that for seasonal influenza (1.3 years). Among their patients with pandemic influenza, age was evenly distributed over the 0–18 years range. They indicated that this finding was consistent with a lack of immunity to the novel virus throughout the pediatric age group [25]. A similar tendency has also been reported in patients with encephalopathy and 2009 pandemic influenza in Japan [20, 26].
Okumura et al. [26] have compared clinical features, laboratory data, neuroimaging findings, treatment, and outcome of acute encephalopathy in historic patients with seasonal influenza and patients with 2009 pandemic influenza. There was no significant difference in neurological symptoms, laboratory and neuroimaging findings, and treatment. However, in patients aged ≥ 6 years, moderate or more severe sequelae were more frequent in patients with 2009 pandemic influenza. They have found that the most prominent difference between the patients with seasonal influenza and 2009 pandemic influenza was the age of the patients. Patients with 2009 pandemic flu had a median age of 109.5 months, which was older than those with seasonal influenza (44 months). Most patients in the 2009 pandemic influenza group were ≥ 6 years old. This could be attributed to a difference in age distribution of the infected patients.
FSs are typically divided into simple and complex types. A simple FS comprises generalized tonic–clonic activity without focal features, of < 10 minutes duration, without recurrence in the subsequent 24 hours and resolving spontaneously. Complex FSs are defined by one or more of the following features: partial (focal) onset or showing focal features during the seizure; prolonged duration (> 10 - 15 minutes); and recurrence within 24 hours or within the same febrile illness [23]. In the present study, simple FSs were seen in 91.1% of patients with 2009 pandemic influenza, 83.8% with seasonal influenza, and 85.7% with non-influenza infection. These differences were not significant.
Central nervous system infections (encephalitis/meningitis) and/or encephalopathy can induce clinical features similar to those of FSs, and it is therefore important to differentiate FSs from those diseases. Close attention should be paid, in particular, to cases with seizures occurring > 24 hours after the onset of fever, and those with atypical seizure as partial seizures, long-lasting seizures of > 15 - 20 minutes duration, and clustering (two or more) seizures within 24 hours [1]. There have only been a few reports on the relationship between onset of fever and seizure occurrence. Chiu et al. [7] have reported that, in 1997, only 3/27 (11.1%) children with seasonal influenza A had FSs at 24 hours after fever onset, and in 1998, 3/54 (5.6%) children with influenza A had FSs at 24 hours after fever onset. In the present study, 30% of patients with seasonal influenza had seizure occurrence at 24 hours after fever onset, which was significantly higher than in influenza A (H1N1) pdm infection (7.8%) and non-influenza infection (10.3%). We found that patients with seasonal influenza had more central nervous system disease, and those with influenza A (H1N1) pdm infection had more respiratory tract disease. The proportion of patients with pandemic (H1N1) 2009 virus infection who developed FSs was 5.1%, which was significantly lower than in those with seasonal influenza virus infection (21.1%). The difference in the proportion of patients who developed FSs caused by pandemic (H1N1) 2009 and seasonal influenza virus infection was supported by a difference in the organs affected in these 2 groups.
The proportion of patients with influenza A (H1N1) pdm infection and first-onset seizure was 45.6%, which was significantly lower than in seasonal influenza (60.0%; P < 0.05) and non-influenza (61.1%; P < 0.01) infection. This could be attributed to a difference in age distribution in the infected patients. We found that FSs occurred at an older age in patients with influenza A (H1N1) pdm infection compared with seasonal influenza and non-influenza infection, and revealed that a low proportion of children with influenza A (H1N1) pdm infection had first-onset seizure.
Seizure duration in patients with influenza A (H1N1) pdm infection did not differ from that in patients with seasonal influenza and non-influenza infection. However, the proportion of patients with FS in influenza A (H1N1) pdm with seizure duration < 5 minutes was lower than that in those with seasonal influenza (P < 0.05) and non-influenza (P < 0.01) infection. These findings showed that the seizure duration in patients with influenza A (H1N1) pdm had a tendency to be longer than those in seasonal influenza and non-influenza infection.
There was no difference in neurological symptoms including seizure manifestations among the patients with 2009 pandemic influenza, seasonal influenza and non-influenza infection in our study.
In conclusion, there were no differences in neurological symptoms when comparing patients with 2009 pandemic influenza, seasonal influenza and non-influenza infection. However, in patients with influenza A (H1N1) pdm infection, FSs occurred in older children compared with seasonal influenza and non-influenza infection. Thus, further detailed studies are required.
Acknowledgments
We thank Dr. Kimiko Ubukata (Graduate School of Infection Control Sciences, Kitasato University, Tokyo, Japan) for performing the real-time RT-PCR of pandemic (H1N1) 2009 virus from nasopharyngeal swabs.
Conflicting Interests
The author(s) declare no potential conflicts of interests with respect to the authorship and/or publication of this article.
Funding
The author(s) received no financial support for the research and/or authorship of this article.
| References | ▴Top |
- Fukuyama Y, Seki T, Ohtsuka C, Miura H, Hara M. Practical guidelines for physicians in the management of febrile seizures. Brain Dev. 1996;18(6):479-484.
pubmed doi - Practice parameter: the neurodiagnostic evaluation of the child with a first simple febrile seizure. American Academy of Pediatrics. Provisional Committee on Quality Improvement, Subcommittee on Febrile Seizures. Pediatrics. 1996;97(5):769-772; discussion 773-775.
pubmed - Ishikawa T, Fuchigami T, Tahara D, Miyashita M, Nakayama Y, Hashimoto K, Inamo Y, et al. Clinical features of influenza-associated febrile seizure (in Japanese, abstract English). J. Nihon. Univ. Med. Ass. 2007; 66(5): 401-404.
- Haruyama W, Fuchigami T, Noguchi Y, Endo A, Hashimoto K, Inamo Y, Fujita Y, et al. The relationship between drug treatment and the clinical characteristics of febrile seizures. World J Pediatr. 2008;4(3):202-205.
pubmed doi - Brocklebank JT, Court SD, McQuillin J, Gardner PS. Influenza-A infection in children. Lancet. 1972;2(7776):497-500.
pubmed doi - Price DA, Postlethwaite RJ, Longson M. Influenzavirus A2 infections presenting with febrile convulsions and gastrointestinal symptoms in young children. Clin Pediatr (Phila). 1976;15(4):361-367.
pubmed doi - Chiu SS, Tse CY, Lau YL, Peiris M. Influenza A infection is an important cause of febrile seizures. Pediatrics. 2001;108(4):E63.
pubmed doi - van Zeijl JH, Mullaart RA, Borm GF, Galama JM. Recurrence of febrile seizures in the respiratory season is associated with influenza A. J Pediatr. 2004;145(6):800-805.
pubmed doi - Stricker T, Sennhauser FH. Complex febrile seizures associated with influenza A. Pediatr Infect Dis J. 2004;23(5):480.
pubmed doi - Hara K, Tanabe T, Aomatsu T, Inoue N, Tamaki H, Okamoto N, Okasora K, et al. Febrile seizures associated with influenza A. Brain Dev. 2007;29(1):30-38.
pubmed doi - Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605-2615.
pubmed doi - Neurologic complications associated with novel influenza A (H1N1) virus infection in children - Dallas, Texas, May 2009. MMWR Morb Mortal Wkly Rep. 2009;58(28):773-778.
pubmed - Tan K, Prerna A, Leo YS. Surveillance of H1N1-related neurological complications. Lancet Neurol. 2010;9(2):142-143.
pubmed doi - O'Leary MF, Chappell JD, Stratton CW, Cronin RM, Taylor MB, Tang YW. Complex febrile seizures followed by complete recovery in an infant with high-titer 2009 pandemic influenza A (H1N1) virus infection. J Clin Microbiol. 2010;48(10):3803-3805.
pubmed doi - Infectious Agents Surveillance Reports. Flash report of influenza virus in Japan, 2009/10 season (seasonal + AH1pdm). 2009. http//idsc.nih.go.jp/iasr/influ-e.html. Accessed August 11, 2011.
- Japan Pediatric Society. Emergency report of updated surveillance data regarding pandemic (H1N1) 2009 infection in Japanese children. (in Japanese) http://www.jpeds.or.jp/influenza/influenza_091113.pdf. Accessed August 11, 2011.
- Hasegawa M, Okada T, Sakata H, Nakayama E, Fuchigami T, Inamo Y, Mugishima H, et al. Pandemic (H1N1) 2009-associated pneumonia in children, Japan. Emerg Infect Dis. 2011;17(2):279-282.
pubmed - Hasegawa M, Inamo Y, Fuchigami T, Hashimoto K, Morozumi M, Ubukata K, Watanabe H, et al. Bronchial casts and pandemic (H1N1) 2009 virus infection. Emerg Infect Dis. 2010;16(2):344-346.
pubmed - Hasegawa M, Hashimoto K, Morozumi M, Ubukata K, Takahashi T, Inamo Y. Spontaneous pneumomediastinum complicating pneumonia in children infected with the 2009 pandemic influenza A (H1N1) virus. Clin Microbiol Infect. 2010;16(2):195-199.
pubmed doi - Imai Y, Ishii W, Endo A, Arakawa C, Kohira R, Fujita Y, Fuchigami T, et al. Influenza-associated encephalopathy due to novel type of influenza A (H1N1) pdm infection in our hospital during the 2009-2010 season (in Japanese, English abstract). J. Nihon. Med. Ass. 2010; 69(4): 224-229.5.
- Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med. 1987;316(9):493-498.
pubmed doi - Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994;35 Suppl 2:S1-6.
pubmed - Waruiru C, Appleton R. Febrile seizures: an update. Arch Dis Child. 2004;89(8):751-756.
pubmed doi - Ostovar GA, Rubin LG, Rajan S, Sood SK, Kohn N. Comparison of the clinical features of children hospitalized with pandemic 2009 A:H1N1 and seasonal influenza. Clin Pediatr (Phila). 2011;50(4):348-354.
pubmed doi - Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361(20):1945-1952.
pubmed doi - Okumura A, Tsuji T, Kubota T, Ando N, Kobayashi S, Kato T, Natsume J, et al. Acute encephalopathy with 2009 pandemic flu: comparison with seasonal flu. Brain Dev. 2012;34(1):13-19.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.