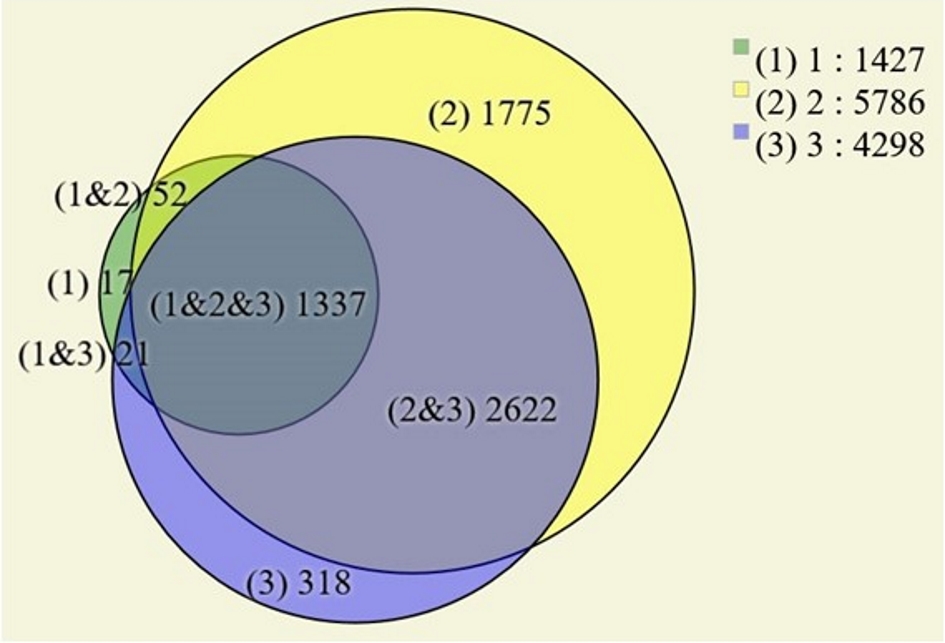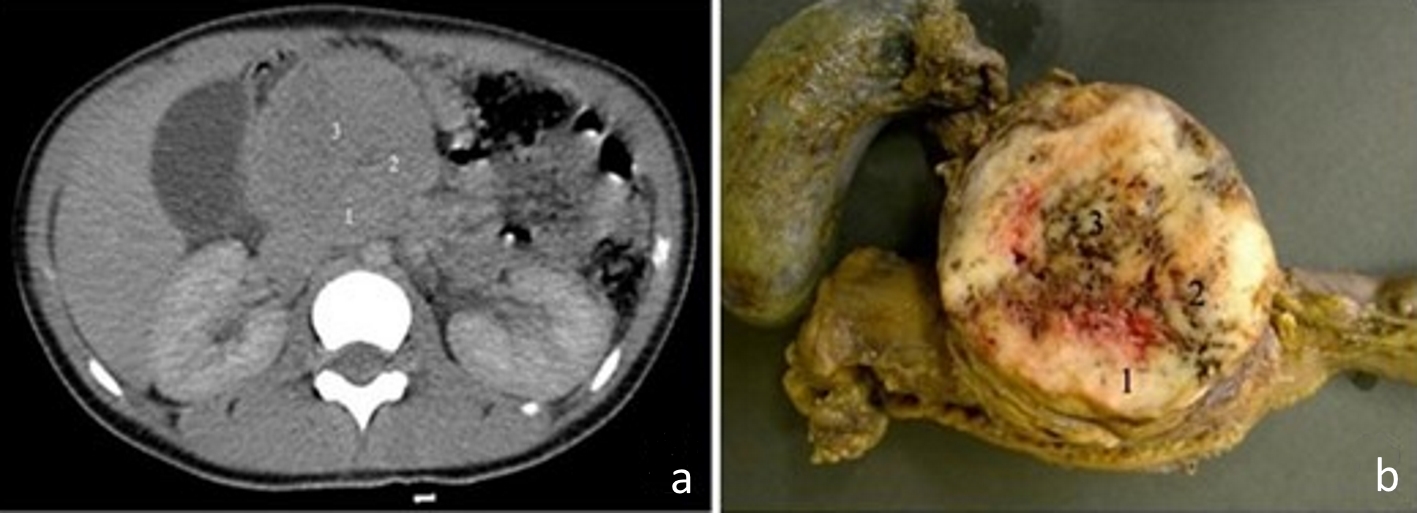
Figure 1. (a) Computed tomography image of the abdomen, in the portal phase, showing a rounded and heterogeneous mass in the head of the pancreas, measuring 6.7 cm (transverse) × 6.3 cm (anteroposterior) × 6.4 cm (longitudinal). Areas 1, 2 and 3 are the topographical areas where fresh tissue samples were collected for the molecular study. (b) Transverse section of solid pseudopapillary neoplasm in the head of the pancreas presenting a solid mass with heterogeneous and whitish aspects, associated with blackened hemorrhagic areas. The numbers represent the regions where fresh tissue samples were collected for the molecular study: 1. peripheral; 2. intermediate; and 3. central.
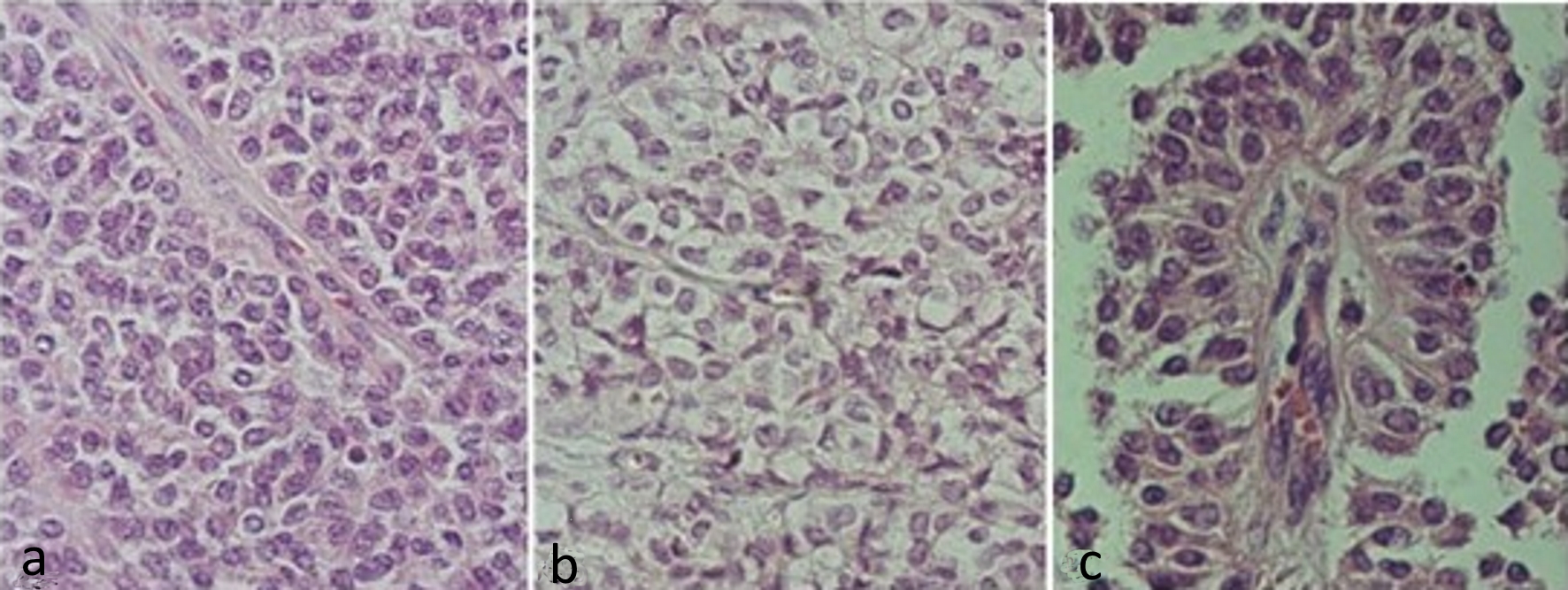
Figure 2. Hematoxylin and eosin stained slides of solid pseudopapillary neoplasm of the pancreas. (a) Solid pattern with barely cohesive cells presenting ovoid or rounded nuclei, sometimes grooved and eosinophilic cytoplasm. (b) Solid pattern showing cells with clear cytoplasm. (c) Pseudopapillary pattern, with cells distributed around vascular connective axis being the nuclei arranged in apical portion.
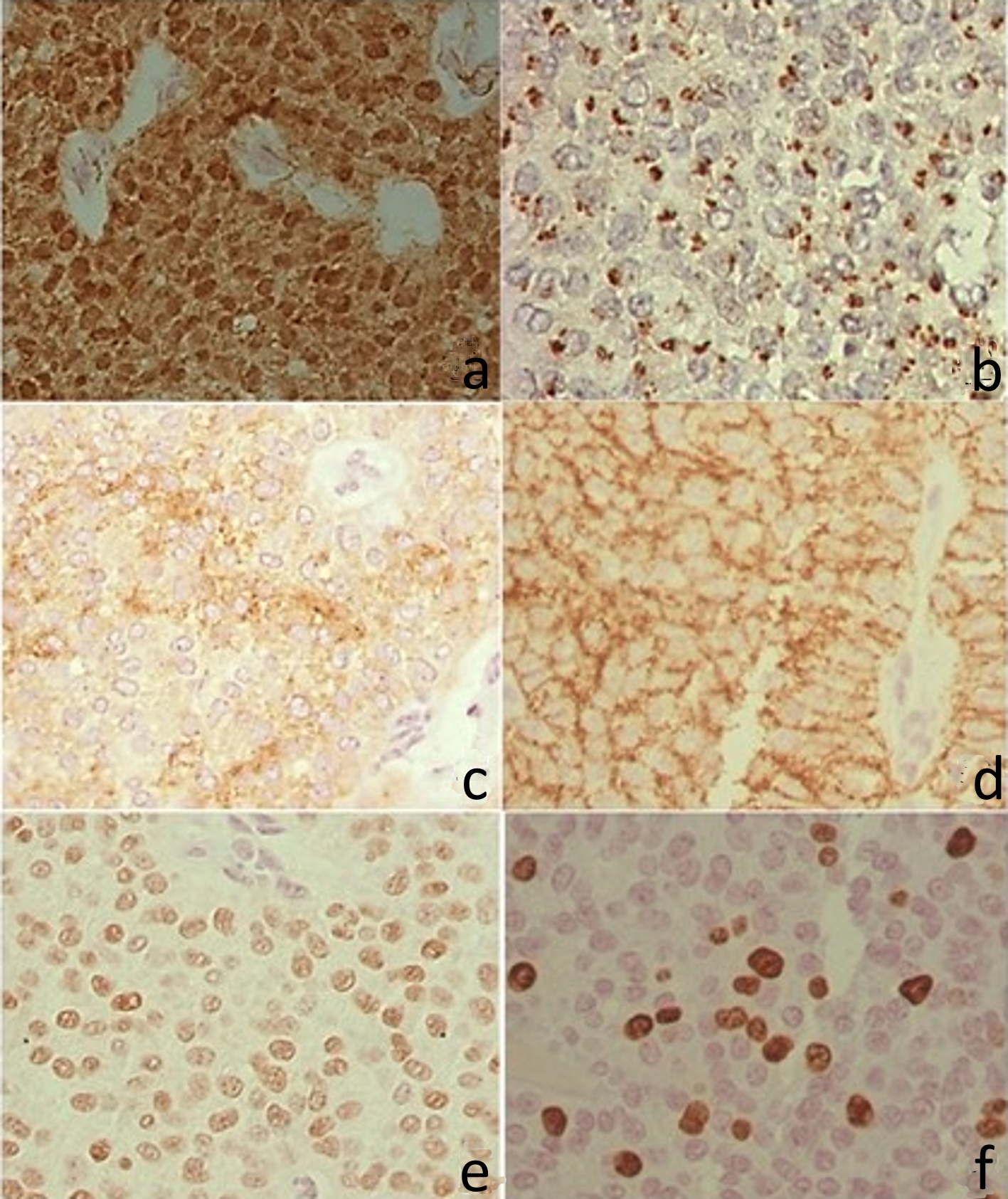
Figure 4. Immunohistochemistry. (a) Positive β-catenin in the nucleus and cytoplasm. (b) CD99 with perinuclear granular marking (dot). (c) CD10 with irregular positivity in the cytoplasm. (d) CD56 with cytoplasmic membrane positivity. (e) Progesterone receptor, with intranuclear labeling. (f) Ki67 with intranuclear labeling.