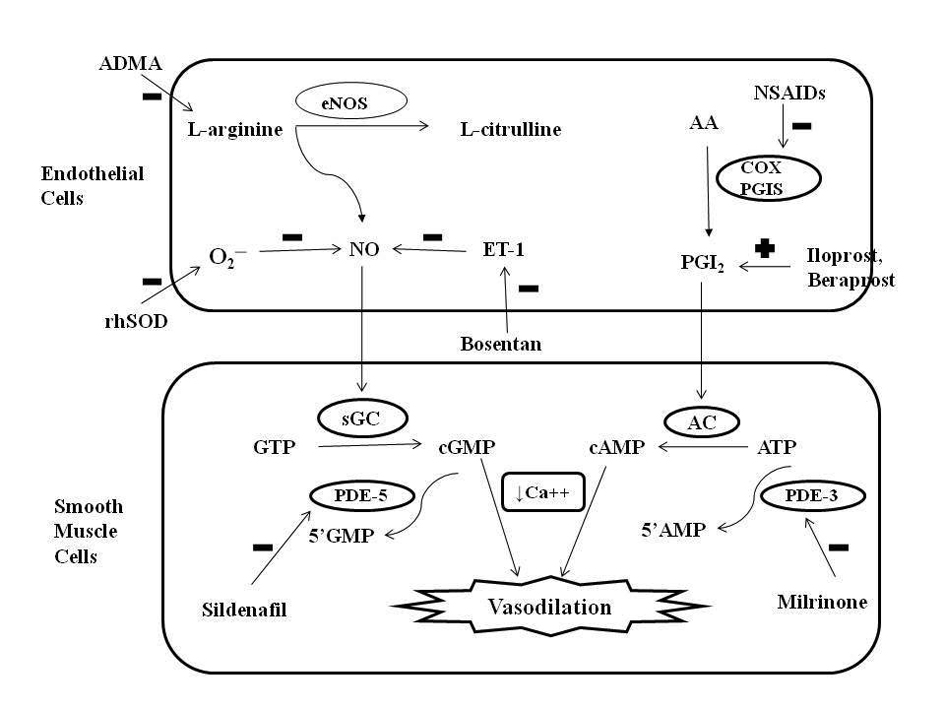
Figure 1. Role of nitric oxide (NO) and prostacyclin (PGI2) signaling pathways in the regulation of pulmonary vascular tone and mechanism of action of different pharmacologic agents. Endothelial NO synthase (eNOS) enzyme stimulates the synthesis of NO by stimulating the conversion of L-arginine to L-citrulline. NO increases intracellular cyclic guanosine monophosphate (cGMP) levels by stimulating soluble guanylate cyclase (sGC) enzyme. PGI2 is an arachidonic acid (AA) metabolite formed by cyclooxygenase (COX) and prostacyclin synthase (PGIS) enzymes in the vascular endothelium. PGI2 stimulates adenylate cyclase in vascular smooth muscle cells, which increases intracellular cyclic adenosine monophosphate (cAMP) levels. Both cGMP and cAMP mediate smooth muscle relaxation by decreasing free cytosolic calcium levels. These cyclic nucleotides are degraded by type 5 and type 3 phosphodiesterase (PDE) respectively thus limiting the duration of vasodilation. Sildenafil and milrinone inhibits PDE-5 and PDE-3 respectively and enhance pulmonary vasodilation. NO levels are decreased by endogenous NO antagonist asymmetric dimethyl arginine (ADMA), superoxide (O2─), and endothelin (ET-1). Exogenous NO administration, endothelin receptor antagonist (bosentan) and rhSOD (recombinant human superoxide dismutase) treatment may promote vasodilation in PPHN by increasing NO levels. Antenatal exposure to non-steroidal anti-inflammatory drugs (NSAIDs) may cause PPHN by interfering with prostacyclin pathway through inhibition of COX. Prostacyclin analogues (PGI2, beraprost sodium, iloprost) may help in PPHN by promoting vasodilation mediated via stimulation of adenylate cyclase. GTP: guanosine triphosphate; ATP: adenosine triphosphate.