| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website http://www.theijcp.org |
Original Article
Volume 7, Number 3, September 2018, pages 21-28
Comparative Study for Probiotics and Symbiotics Versus Placebo in Pediatrics Acute Diarrhea: Randomized Controlled Trial
Pamela Houeissa, Antoine Faraha, Peter Nouna, Mode AlOjaimib, Georges AbiFaresa, Myriam Amma, Hala Feghalia, Georges Nicolasa, Gisele Nadera, Marie Samarania, Peter Makhoulc, Marie Claude Fadous Khalifea, d
aHoly Spirit University of Kaslik, Kaslik, UH-Notre Dame Des Secours, Byblos, Lebanon
bBalamand University, Koura, Familial Medical Center, Zgharta, Lebanon
cUniversity of Masachusetts, Amherst, USA
dCorresponding Author: Marie Claude Fadous Khalife, Holy Spirit University of Kaslik, Kaslik, UH-Notre Dame Des Secours, Byblos, Lebanon
Manuscript submitted January 31, 2018, accepted March 27, 2018
Short title: Probiotics and Symbiotics in Acute Pediatric Diarrhea
doi: https://doi.org/10.14740/ijcp288e
| Abstract | ▴Top |
Background: Acute diarrhea is a major cause of morbidity and mortality in children, particularly in developing countries (the second cause of death). Probiotics and symbiotics are recent treatments for this disease, especially in the acute phase. Our objective is to compare probiotic or symbiotic treatment against placebo in acute diarrhea by following the evolution of diarrhea in terms of hours, number of bowel movements, volume of stools and their consistency. On a larger scale, we want to find a cost-effective intervention that reduces the morbidity and mortality of diarrhea.
Methods: Eighty-three children aged 6 months to 5 years, from three different regions of Mount Lebanon, were randomized to receive a probiotic, a symbiotic or a placebo once daily for 5 days. Patients were excluded from the study if they had any history of a chronic disease. The statistical analysis was carried out on SPSS v22.00
Results: Out of 120 surveys distributed to parents, 84 were completed: 43 patients received probiotics (nine received Lactobacillus, 21 received spores, and 13 patients received yeast), 24 received symbiotics and 17 were controls. Stool consistency normalized on day 4 in the probiotics and symbiotics groups (P = 0.009). Less number of days with fever (P = 0.018) were observed in the probiotic and symbiotic groups (1 day) compared to placebo (4 days). No difference in the symptoms associated with diarrhea was observed in the different groups.
Conclusions: Probiotics and symbiotics normalized stool consistency in pediatric diarrhea by day 4 and decreased the number of days with fever compared to control. Our study did not show a statistically significant difference between the different probiotics and symbiotics for the treatment of diarrhea.
Keywords: Acute diarrhea; Children; Probiotics; Symbiotic
| Introduction | ▴Top |
Gastroenteritis is a major health burden worldwide; approximately two billion cases are reported each year [1]. It causes significant morbidity and mortality (the second leading cause of death), particularly in developing countries [2]. The majority lasts for an average of 2 to 3 days. The pathophysiology of diarrhea is described as an interruption of the enterosystemic cycle of water which is responsible for the loss of water and electrolytes leading to dehydration in the absence of replacement therapy. Oral rehydration solutions compensate for the losses but will not change the stool volume or consistency and will not normalize the gastrointestinal flora [3]. Anti-diarrheal drugs, used usually in adults, decrease the intestinal transit [4]. They are not advised in children before 2 years of age because of the risk of central nervous effects (respiratory depression and coma) and ileus. Since the main goal of treatment is to shorten the duration of diarrhea and to reduce the morbidity while avoiding side effects [5]. Other solutions are sought in pediatrics based on studies of the ubiquitous intestinal flora. Among these solutions is the use of probiotics and symbiotics [6].
Probiotics consist of intestinal microorganisms [7]. Many studies are focusing on their efficacious role in replenishing the gut microbiota after an intestinal insult. Gibson and Roberfroid introduced the “probiotic” term. It refers to an “additive or non-digestible ingredient, mainly polysaccharides that affects the host by selectively stimulating the growth or activity of non-pathogenic bacteria” [8]. Symbiotics are a combination of both prebiotics and probiotics. Few studies compared the efficacy of symbiotics vs. probiotics in diarrhea.
The majority of the studies compared the symbiotics to a placebo. In this trial, we compare the effect of probiotics, symbiotics and placebo on acute diarrhea in children by studying their effect on the stool’s frequency and consistency, on the duration of diarrhea and on the associated symptoms. On a larger scale, we are looking for a cost-effective solution to reduce the morbidity and mortality from diarrhea.
| Methods | ▴Top |
Study design
We conducted a prospective, randomized, double-blind, placebo-controlled, multicentred study by collecting data from questionnaires completed by parents and physicians.
Our study lasted from September 2014 to April 2015(off diarrhea season). It took place in three private pediatric clinics in Lebanon and subsequently included 84 children from different parts of Mount Lebanon (Jdeideh-Jeita-Jbeil).
Acute diarrhea was defined as per WHO by the emission of at least three loose or watery stools per day. Pediatricians evaluated the symptoms and the degree of dehydration of the children and prescribed appropriate treatment. Among the options were antibiotics, oral rehydration solutions, IV hydration, anti-diarrheal medications, zinc and one of the proposed drugs (probiotics, symbiotics or placebo) (Table 1). Exclusion criteria were any chronic intestinal disease such as celiac disease, cystic fibrosis, food intolerance, immunodeficiency, IBD, gastrointestinal malformations, intestinal motility disorder, daily oral probiotic or symbiotic use, uncooperative parents in the follow-up of the child and any secondary diarrhea: bronchiolitis, pneumonia, ENT infection etc. The parents signed a written informed consent. Laboratory tests were performed when clinically indicated.
 Click to view | Table 1. Samples Used for Treatment |
The study population consisted of all children between 6 months and 5 years of age presenting to one of the three private clinics for acute diarrhea (Fig. 1).
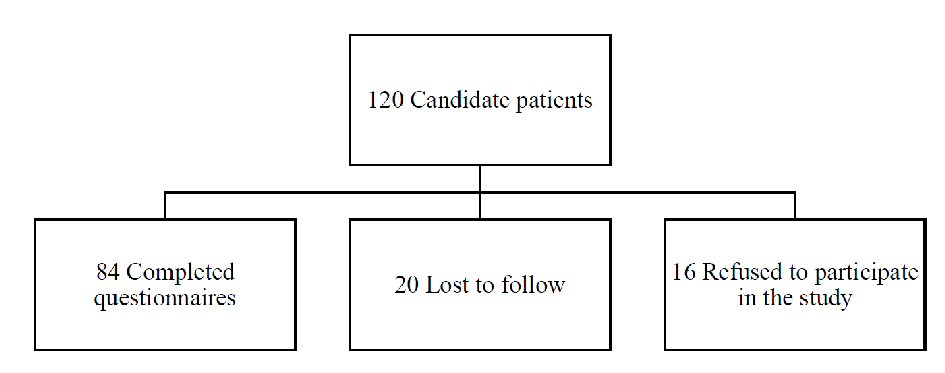 Click for large image | Figure 1. Consort flow diagram. |
Intervention
The children were divided randomly into five groups; the first group received Lactobacillus (Lactobacillus acidophilus and Bifidobacterium lactis); the second group received Bacillus clausii; the third group received a yeast (Saccharomyces boulardii); the fourth group received a symbiotic (Bacillus coagulans + fructo-oligosaccharides) and the last group received placebo (Table 1). All of these drugs were packed, numbered and put in a box by the researcher. Each bag contained five capsules to be given once daily over 5 days. The capsules were opened before being administered. The clinician has randomly drawn a sample and the number of the sample was documented in the questionnaire.
The parents received a paper for charting; they had to document the number of episodes of diarrhea per day, the timing at which each episode occurred, the consistency of the stools and the associated symptoms (vomiting, fever and abdominal pain). The diarrhea was considered resolved when the child had stools of normal consistency. The selection of probiotics and symbiotics in this study was based on the most studied supplements in the literature. They have been shown to be effective in multiple studies without any adverse effects. The choice of the variables to be studied was based on the systematic review (Measurement Issues in Trials of Pediatric Acute Diarrheal Diseases) and on the results of clinical studies on these microorganisms. The stool consistency was based on the Bristol scale of stool shape used by Vandenplas. The duration of treatment and the doses administered were applied as recommended. The treatment received by each child remained unknown until the statistical analysis was completed.
The samples used for treatment were provided free of charge to encourage the cooperation of the parents. Statistical analysis of the results was performed using the SPSS program version 22.00 (SPSS Inc., Chicago, IL); the table had continuous and discrete variables; the data series had a two-dimensional table structure: one for patients and the other for different variables. The discrete variables were analysed by the Chi-square method, the continuous variables by the t-test and ANOVA. A P value of 0.05 or less was considered significant. We compared probiotics to symbiotics, intervention group to placebo and the five groups.
| Results | ▴Top |
In our study, 120 children presented for acute diarrhea and met the inclusion criteria. Sixteen parents refused to participate for fear of receiving a placebo. A total of 104 children participated in the study, 20 children were lost to follow-up. The patients were divided into five groups: nine in group 1, 21 in group 2, 13 in group 3, 24 in group 4 and 17 patients received placebo. Demographic characteristics were comparable. The sample was normally distributed. At baseline, the degree of dehydration was similar in the five subgroups. No children were excluded from the study.
The percentage of males was 48.8% and that of females is 51.2%. The average age was 24.7 months. The mean weight in the probiotic group was 13.69 kg, 13.02 kg in the symbiotic group and 15.19 kg in the placebo group (Table 2). Children presented to the clinics on average of 2.5 days from the onset of diarrhea and the majority were not dehydrated (< 5%). Parents or physicians have reported no side effects.
 Click to view | Table 2. Contingency Table: Sample Results |
There was no difference between the probiotic and symbiotic group in terms of diarrhea duration in days and hours with a P value of 0.37 and 0.61, respectively. However, it should be noted that the average duration of diarrhea in the probiotic group (76.93 h and 4.05 days) was higher than that of the symbiotics (70.43 h and 3.65 days).
Stool consistency normalized mainly at day 4 in the group of probiotics and symbiotics with P = 0.909. By following the daily changes in the number of watery stools, we noted a constant decrease in the probiotic group more than the symbiotic group but these results were not significant. In terms of associated symptoms, no significant changes were noted between the two groups.
In the intervention group, the average days of diarrhea following treatment with probiotics and symbiotics were 3.91vs. 4.35 in the placebo group. This difference is not significant according to the independent t-test with a P value of 0.33. Similarly, the comparison of the placebo group with the other groups according to the daily number of episodes of diarrhea on the first 3 days as well as the number of hours of diarrhea, days of vomiting and days of fever showed a speedier recovery in the group receiving probiotics and symbiotics versus placebo. These results were not significant. A significant relationship was observed between the three groups and the consistency of stools at day 4; the children who received a symbiotic or a probiotic had a normal consistency at day 4 more frequently than those who received placebo with a P < 0.05 (0.001).
By comparing all five groups, the consistency at day 2 is found to be abnormal in all groups and the Fisher exact test confirmed that there was no significant difference between the five groups of patients tested for the different parameters observed.
By comparing the three groups: probiotics, symbiotics and placebo, the only significant finding was the consistency at day 4 post treatment. Using the ANOVA test, the reduction in the number of episodes of diarrhea between the first day and the fourth day of diarrhea was studied; diarrhea decreased mainly in the group that received the symbiotics and to a lesser extent in the group that received the probiotics. The placebo group had the least decrease in the number of episodes of diarrhea. This decrease was not significant. This is also observed with the associated symptoms (number of days of fever, number of days of pain) without it being significant. So there is no significant difference between the three groups of people tested for the different parameters. On the other hand, the consistency of stools became normal on day 4 with probiotics and symbiotics, whereas children receiving placebo always had a soft or fluid consistency with P = 0.009.
When we divide the sample into probiotic, symbiotic and placebo, our statistics showed a significant result concerning the number of diarrheal episodes at day 4. We have a significant relationship between placebo and probiotic on one hand and between placebo and symbiotic on the other hand, with a P value of 0.002 and P value of 0.026, respectively. The analysis suggested an improvement in stool average from 3.37 episodes in placebo group to 1.5 episodes in symbiotic group to one episode in probiotic group (Fig. 2).
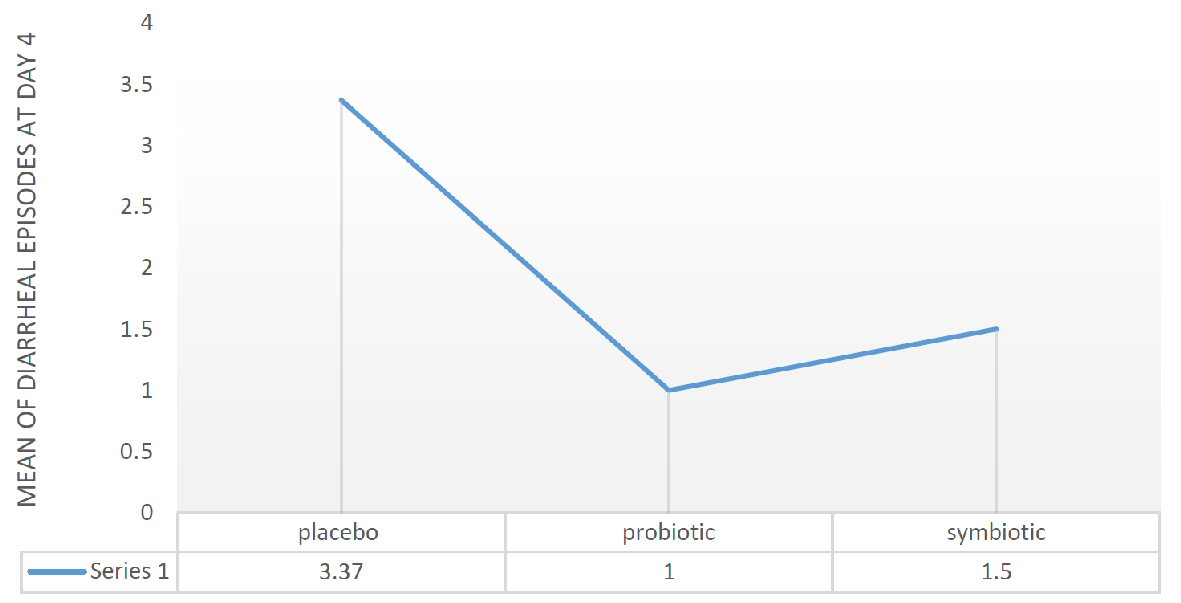 Click for large image | Figure 2. Mean diarrheal episodes at day 4 in placebo, probiotic and symbiotic group. |
This result is also observed for the number of days of fever with a P value of 0.026 (independent t-test). In the placebo group, the average days of fever are 4, whereas they are limited to 1 day in the other two groups with P = 0.018 and 0.02 respectively (Fig. 3).
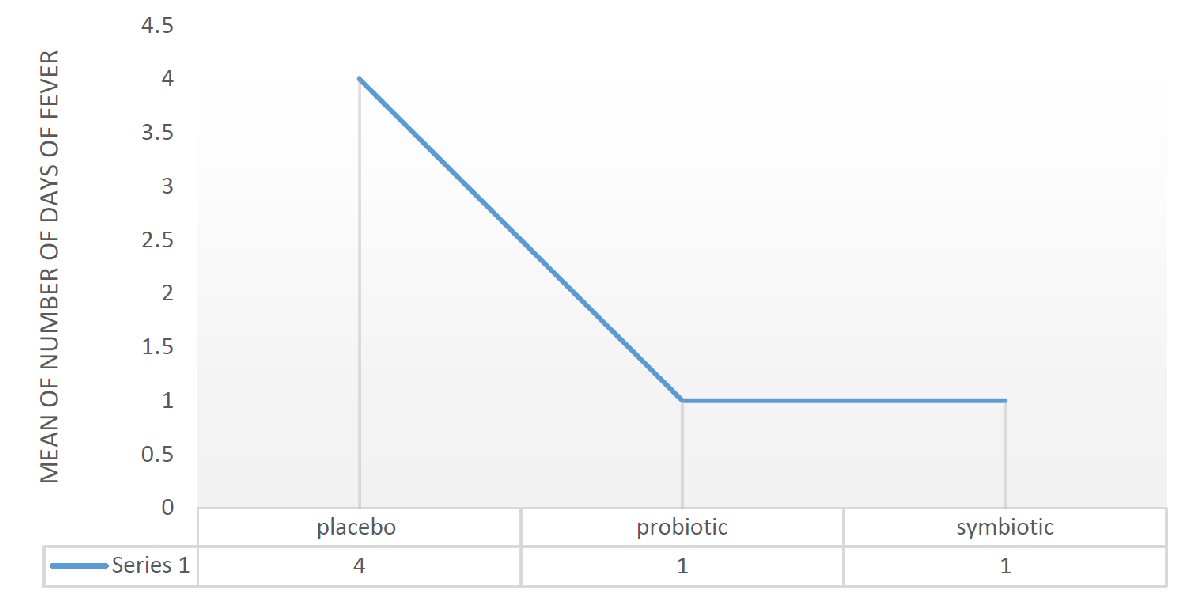 Click for large image | Figure 3. Mean of days of fever in placebo, probiotic and symbiotic group. |
If we compared the five groups, the one-way ANOVA showed a significant result (0.012) regarding the number of diarrheal episodes at day 4. But what can be said is that the average stools are 3.37 in the placebo group whereas in the other groups they are between 1 and 1.5 with the least value for the Lactobacillus group (group 1). Number of febrile days is 4 on average in the placebo group while they are limited to 1 - 1.5 days in the other groups with a P value of 0.012. The results of duration of diarrhea, mean episodes of diarrhea at day 4 and number of febrile days when comparing any type to placebo are provided in Figure 4.
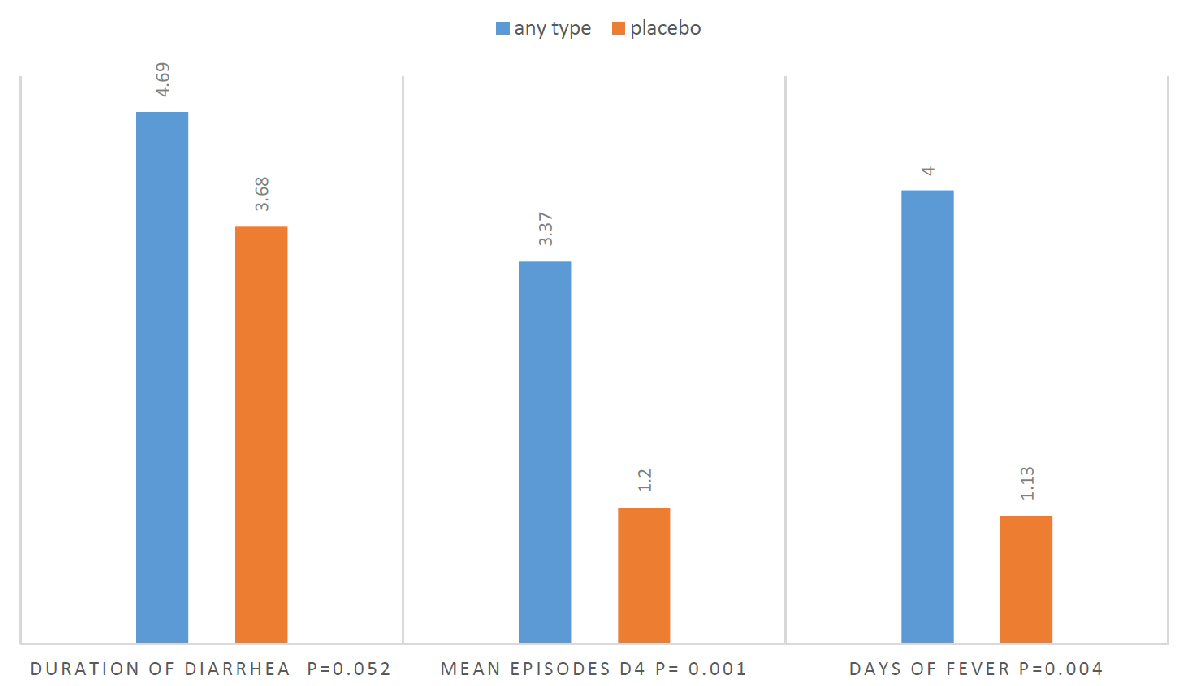 Click for large image | Figure 4. Independent t-test comparing any type to placebo. |
Delay for initial treatment, degree of dehydration, characteristic of diarrhea showed no effect on diarrheal resolution. Only the number of stools per 24 h before treatment had a role on the stool consistency at day 4 (P = 0.013). Consistency at day 4 normalized earlier in the group that had fewer than three diarrheal stools before treatment. Diarrhea less than three stools/24 h is associated with less febrile days than diarrhea greater than three stools/24 h upon presentation with P = 0.013.
Concerning the number of diarrheal stools at day 4 according to the treatment (Fisher exact test), the probiotic allowed a greater reduction of the diarrheal stools at day 4 (0.5) compared to the ORS group alone (3.83) in the children presenting after 2 days of diarrhea with a P = 0.003.
According to the independent t-test, fever days in the same category (children presenting after 2 days of diarrhea) were less among probiotics or symbiotic (1.36 days) versus 2.82 days in the placebo group (P = 0.005). The consistency at day 4 was always normal in the probiotic and symbiotic groups, not in the placebo group with P = 0.008, P = 0.004 and P = 0.0029.
| Discussion | ▴Top |
The intestinal flora protects the digestive tract against infections. A change in this flora leads to an imbalance that is manifested by digestive pathologies and in our case, diarrhea. Diarrhea is usually self-limiting, but can lead to significant morbidity and mortality by causing dehydration. The hypothesis is that probiotics and symbiotics associated with ORS can hasten cure by acting on both the immune system and intestinal flora and thus by acting on the pathophysiology of diarrhea.
Many studies have investigated the efficacy of probiotics in the treatment of acute diarrhea, but there is still no consensus regarding the definition of acute versus resolving diarrhea, as well as in the strains and doses of probiotics that should be administered. Studies on probiotics have increased in recent years. Many studies looked at the benefits, and others wanted to determine the effective dose. The heterogeneity in the studies resulted in different results concerning management of diarrhea. Studies show better effect of probiotics on viral gastroenteritis than on bacterial or parasitic infections. The mechanisms of action are linked to the strains used [9]. There is evidence regarding the efficacy of lactobacilli strains (e.g. Lactobacillus casei GG and Lactobacillus reuteri ATCC 55730) and Saccharomyces boulardii in diarrhea patients [7]. Many factors influence the outcome: diet, environment, genetic factors, vaccination, age etc. The interval of administration seems also important. In our study, the sample was distributed across Lebanon to obtain reliable results regardless of demographic characteristics.
The effectiveness of a probiotic depends on its interaction with the specific flora of the host or with the intestinal immune system [10]. The development of the immune system after birth depends largely on the development and composition of the intestinal flora and vice versa [11]. Many studies have been carried out and have proved the action of probiotics in the protection against pathogens by immunostimulation, and this is why their use in clinical trials has reduced symptoms in terms of duration (1 to 1.5 days), increased anti-rotavirus antibody production with strains Lactobacillus rhamnosus GG, L. casei Shirota, L. reuteri, and Bifidobacterium lactis. Small children are more susceptible to probiotics due to their immature immune system and the simplicity of their intestinal flora [11].
There were 20 studies out of 2,751 conducted showed that probiotics decrease the duration of diarrhea to 4 days and decrease its frequency on day 2 [12]. In our study this is not the case. On the contrary, the number of diarrheal stools is maximal at day 2 in the five groups and the improvement is noted at day 4 but also in the five groups. A reduction of 0.7 - 1 day (24 h) in the group receiving probiotics, especially lactobacilli, was often reported in the literature. Few studies investigated the action of symbiotics, while multiple studies explored the action of prebiotics. The different results obtained can have several explanations.
It is recognized that probiotics and prebiotics are present in functional foods [13] and subsequently a child in the control group with diarrhea receiving a diet containing these adjuvants may show the results as intervention group patients. This might explain that no difference in results was found between any pre/probiotic type and placebo. In a French prospective randomized study, 287 children were followed in nurseries. They were divided into three groups and received consecutively milk, yoghurt and a probiotic containing 108 cfu/mL casei. Each product was administered for 1 month. Yogurt decreased the average duration of diarrhea by 5 to 8 days and the probiotic was 4.3 days (P = 0.01).
Few studies compared the effect of probiotics and symbiotics on acute diarrhea in children. A study in Java-Indonesia, conducted on 188 children randomly assigned to receive a probiotic or a symbiotic, showed no difference between these two groups in terms of duration of diarrhea and frequency of diarrheal stools per day. The absence of difference between probiotics and symbiotics can be explained by the fact that prebiotics need 10 to 14 days to act; hence, they are mainly used for prevention. Similarly, the fructo-oligosaccharides (FOS) strengthen the microbial flora and may prevent the multiplication of pathogens, but the action of FOS in the reduction of diarrhea remains unclear. Fermentation of oligofructose in the colon increases the number of Bifidobacterium in the colon [14], increases fecal weight, and shortens the gastrointestinal transit time. Oli et al has shown that the addition of FOS to an oral solution accelerates the recovery of the flora in an animal model [15]. A randomized double-blind clinical study investigated the action of oligofructose in acute diarrhea of newborns and infants. A significant improvement in the severity of the disease that is manifested by the fever, the need for medical consultation and the absenteeism in daycare centers is observed [16-18]. A study in Bangladesh on 150 children randomly receiving FOS for 6 months showed reduced episodes of diarrhea and duration of episodes per day [14]. Oli et al [15] showed an acceleration of healing with the association of FOS and oral hydration solutions in animals. Brunser et al studied the effect of FOS on microbial flora in children treated with amoxicillin; an increase in Bifidobacterium was observed in the prebiotic group [19]. A preventive effect of common digestive disorders was reported by Saavedra in the study on 140 infants receiving FOS; they had significantly fewer episodes of vomiting, regurgitation and abdominal discomfort (judged by parents) but also fewer fevers, less medical complications and fewer antibiotic intakes [20].
Therefore, the lack of difference in efficacy between probiotics and symbiotics may be due to the fact that prebiotics (FOS) require 14 days to act on the microbial flora. Thus, supplementation of children with prebiotics in acute diarrhea will have no effect on the stools during the first 10 days of treatment. Therefore, they are best in prevention than treatment [21, 22]. Another possible explanation is that children already have the probiotic substrates in their digestive system and therefore they do not benefit from this mixture (probiotic and symbiotic) so symbiotics may be more effective in malnourished children or in cases of severe diarrhea associated with weight loss [23].
There was no difference in the time required for resolution of diarrhea between the different probiotics studied. This may be due to the fact that all the strains used are efficacious on shortening the duration of diarrhea and decreasing the stool’s frequency. Studies on the various strains (Lactobacillus, Saccharomyces boulardii, Bifidobacterium) have often shown a reduction of diarrhea duration around 24 h and a significant decrease in diarrheal stool frequencies from day 2 and a resolution of diarrhea at day 3 and day 4 [24-26].
Despite the heterogeneity in the studies, common points were noted: probiotics have no side effects; they reduced the duration of diarrhea on average 24 h (result of 35 studies on 4,555 patients) and L. GG and S. boulardii had a constant efficacy in the studies [27]. The heterogeneity in the results may be secondary to the methodology but also to other factors such as probiotic strain, strain number, live or killed microorganisms, dosage, rotavirus infection and severity of diarrhea [28]. Statistical heterogeneity is expected in view of the diversity of diarrhea definitions and cure criteria, in relation to the probiotics studied, management, etiology and study setting. A meta-analysis has shown that heterogeneity continues to be present even in stratification cases under the conditions noted previously [25-28]. This heterogeneity in the studies weakens the “evidence base” [29], which is why studies with a large sample and a precise management protocol and more objective measurement methods are needed. It is also necessary to divide the sample into subgroups according to the elements already mentioned which may change the results. In our study, the age, sex, degree of dehydration, and characteristics of diarrhea did not affect the course of the disease. On the other hand, the frequency of diarrhea at presentation had an effect on the number of febrile days, stool frequency and consistency at day 4. Thus a child presenting with fewer than three diarrheic bowel movements had less severe diarrhea and thus a faster healing.
A modified Vesikari score was used to assess pediatric diarrhea. The analysis showed that, after excluding cases of moderate and severe diarrhea (score > 8), the effectiveness of probiotics and symbiotics remained unchanged [30].
Rotavirus vaccination and weight loss have played a role in accelerating or slowing down the healing process [30]. Thus, by limiting our sample to children vaccinated against rotavirus, the results have changed; an increase in the response to probiotics and symbiotics is observed. The oral rotavirus vaccine associated with Bacillus clausii increases the response by increasing specific IgMs and sero-conversion of IgAs. Probiotics potentiate the immune reactions triggered by the vaccine, subsequently amplify the immune response against the antigens of the viruses and thus promote the body’s defense against them. A study of pigs [31] showed that the group receiving the two doses of rotavirus oral vaccine with Lactobacillus acidophilus had an increase in rotavirus-specific CD8 LT in the ileum and spleen and in the rate of IgG and IgA lymphocytes, as well as an amplified secretion of IgG, IgA, IgM and neutralizing antibody in serum compared to the group only receiving the vaccine.
In the vaccinated sample, probiotics and symbiotics were better than placebo. A reduction of two episodes and three episodes of stools was obtained on day 4 with symbiotics and probiotics respectively without one being better than the other. Similarly, a reduction in the number of days of fever and therefore severity was observed with probiotics and symbiotics. By comparing the five groups, the stool frequency at day 4 in group 1 (Lactobacillus acidophilus and Bifidobacterium lactis) was reduced by 2.3 episodes compared to placebo. Microorganisms have been shown to be effective by reducing the number of days of diarrhea by 1 day and the number of stools by 40 h compared to the placebo group. Subsequently, vaccination against rotavirus increased the antidiarrheal effect of probiotics and symbiotics. In view of these results, vaccination against rotavirus in developing countries, especially in Africa, can be proposed to limit secondary mortality to diarrhea, despite the risk of investigation on the basis of the risk/benefit principle.
Similarly, weight loss had an effect on the consistency of the stool and eventually resulted in a decrease in the effectiveness of probiotics or delayed healing. This has played a role in changing the results of the study. Weight loss is a criterion of severity; it can lead to malnutrition and thus a period of time for recovery.
Treatment started 2 days after the symptoms’ onset made probiotics and symbiotics more effective on stool frequency and consistency at day 4. Perhaps this is the natural history of the disease. But if it was the case, it would have given the same results in the placebo or the isolated oral hydration solution group. In our study, the microorganisms coupled to the ORS were more effective in reducing the number of stools on day 3. Similarly, the probiotic group compared to the placebo group improved the stool frequency at day 3. So when treatment is started 2 days or more after the onset of diarrhea, probiotics will act and reduce the number of diarrheal episodes from the third day. These results are opposite of the published literature which suggests that the early onset of treatment has a better yield of recovery.
The effectiveness of probiotics is also influenced by etiology. These microorganisms are mainly effective in diarrhea of viral origin, especially rotavirus, and may sometimes require an association with antibiotic therapy for better action. An example is a study showing that the association of probiotic-metronidazole has a better action than metronidazole alone in diarrhea [32]. Usually, the etiology determines the management.
A study of Saccharomyces boulardii has shown that probiotics are effective regardless of etiology, but to different degrees [33]. Other studies have insisted on the efficacy of probiotics demonstrated in rotavirus gastroenteritis but are still evaluating their efficacy in other gastroenteritis, especially that the strains of probiotics have a specificity not only in their mechanism of action but also on their target [34]. This is one of the causes of the heterogeneity observed in randomized clinical studies. For example, Lactobacillus strains have a modest but significant effect on acute diarrhea [35]. Lactobacillus GG is more effective on rotavirus and immunomodulation, Lactobacillus acidophilus on antibiotic-associated diarrhea, L. casei and L. reuteri on rotavirus, B. lactis on rotavirus, travellers’ diarrhea and oral vaccination, and Saccharomyces boulardii is particularly useful in antibiotic-associated diarrhea (Clostridium difficile) and IBD [36]. Sometimes, it is not enough to give probiotics; you have to treat the underlying cause [13]. So probiotics will be adjuvants to antibiotics, hydration solutions and diet.
Zinc was used in our study as a treatment option but it showed no benefit on diarrhea. Its effectiveness is not yet proven. It is a subject of study in developing countries. At present, it is resulting in a 25% reduction in diarrhea and 30% in stool volume [9].
The results of our study were comparable to some studies and inconsistent with others. We are though aware that we cannot extrapolate the results obtained in other countries and apply them to Lebanon.
The strains used and the doses administered are not adapted to our lifestyle. The difference can be due to breastfeeding, eating habits, climate, composition of the intestinal flora of the Lebanese population and the percentage of rotavirus infection.
A weakness of the study is the limited number of the sample especially as the study was conducted outside the diarrhea season. In addition, the evaluation of diarrhea and its evolution was partly subjective; it was made by the parents and thus can vary from one case to another. Similarly, we couldn’t ascertain the compliance of the children to the treatment and the diet of the child was not analysed during diarrhea.
Conclusions
Our data shows that pediatric patients with acute diarrhea given probiotics or symbiotics had normal stool consistency on day 4 of illness. No statistical significant difference was observed between treatments with the symbiotics and probiotics for acute pediatric diarrhea, so symbiotics were not found to be superior to probiotics. Further studies on a larger sample of patients are required in order to see if these results could be replicated or duplicated.
Conflict of Interest
The authors declare no conflict of interest.
| References | ▴Top |
- Farthing M, Salam M, lindbergG. La diarrhee aigue chez les adultes et les enfants: une approche globale. World Gastroenterology Organisation. 2012.
- Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE, Child Health Epidemiology Reference Group of the World Health O, et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8(9):e72788.
doi pubmed - Afshan Shafi, Umar Farooq, Kashif Akram, Mahgul Jaskani, Farzana Siddique, Amna Tanveer. Antidiarrheal effect of food fermented by various strains of lactobacillus. Comprehensive Reviews in Food Science and Food Safety. 2014;13(2):229-239.
doi - Buda B, Dylus E, Gorska-Fraczek S, Brzozowska E, Gamian A. [Biological properties of Lactobacillus surface proteins]. Postepy Hig Med Dosw (Online). 2013;67:229-237.
doi - Vandenplas Y, De Hert S, Probiotical study g. Cost/benefit of synbiotics in acute infectious gastroenteritis: spend to save. Benef Microbes. 2012;3(3):189-194.
doi pubmed - Salminen S, Nurmi J, Gueimonde M. The genomics of probiotic intestinal microorganisms. Genome Biol. 2005;6(7):225.
doi pubmed - C HC, T RK. Probiotic potency of Lactobacillus plantarum KX519413 and KX519414 isolated from honey bee gut. FEMS Microbiol Lett. 2018;365(4).
- Cui S, Hang F, Liu X, Xu Z, Liu Z, Zhao J, Zhang H, et al. Effect of acids produced from carbohydrate metabolism in cryoprotectants on the viability of freeze-dried Lactobacillus and prediction of optimal initial cell concentration. J Biosci Bioeng. 2018;125(5):513-518.
doi pubmed - Mammas IN, Greenough A, Theodoridou M, Kramvis A, Rusan M, Melidou A, Korovessi P, et al. Paediatric Virology and its interaction between basic science and clinical practice (Review). Int J Mol Med. 2018;41(3):1165-1176.
doi - Amer M, Nadeem M, Nazir SUR, Fakhar M, Abid F, Ain QU, Asif E. Probiotics and their use in inflammatory bowel disease. Altern Ther Health Med. 2017:pii:AT5641.
pubmed - Nash MJ, Frank DN, Friedman JE. Early Microbes Modify Immune System Development and Metabolic Homeostasis-The "Restaurant" Hypothesis Revisited. Front Endocrinol (Lausanne). 2017;8:349.
doi pubmed - Braga VL, Rocha L, Bernardo DD, Cruz CO, Riera R. What do Cochrane systematic reviews say about probiotics as preventive interventions? Sao Paulo Med J. 2017;135(6):578-586.
doi pubmed - Shi Y, Zhao X, Zhao J, Zhang H, Zhai Q, Narbad A, Chen W. A mixture of Lactobacillus species isolated from traditional fermented foods promote recovery from antibiotic-induced intestinal disruption in mice. J Appl Microbiol. 2018;124(3):842-854.
doi pubmed - Cherbut C. Prebiotiques et fonctions gastro-intestinales: revue des effets et des perspectives. Cah Nutr Diet. 2003;38(6):346-354.
- Oli MW, Petschow BW, Buddington RK. Evaluation of fructooligosaccharide supplementation of oral electrolyte solutions for treatment of diarrhea: recovery of the intestinal bacteria. Dig Dis Sci. 1998;43(1):138-147.
doi pubmed - Bodera P. Influence of prebiotics on the human immune system (GALT). Recent Pat Inflamm Allergy Drug Discov. 2008;2(2):149-153.
doi pubmed - de Vrese M, Marteau PR. Probiotics and prebiotics: effects on diarrhea. J Nutr. 2007;137(3 Suppl 2):803S-811S.
doi pubmed - Barcelo A, Claustre J, Moro F, Chayvialle JA, Cuber JC, Plaisancie P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46(2):218-224.
doi pubmed - Brunser O, Gotteland M, Cruchet S, Figueroa G, Garrido D, Steenhout P. Effect of a milk formula with prebiotics on the intestinal microbiota of infants after an antibiotic treatment. Pediatr Res. 2006;59(3):451-456.
doi pubmed - Saavedra JM, Abi-Hanna A, Moore N, Yolken RH. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr. 2004;79(2):261-267.
doi pubmed - Boehm G, Moro G. Structural and functional aspects of prebiotics used in infant nutrition. The Journal of Nutrition. 2008;138(9):1818-1828.
doi pubmed - Schrezenmeir J, Heller K, McCue M, Llamas C, Lam W, Burow H, Kindling-Rohracker M, et al. Benefits of oral supplementation with and without synbiotics in young children with acute bacterial infections. Clin Pediatr (Phila). 2004;43(3):239-249.
doi pubmed - Passariello A, Terrin G, Cecere G, Micillo M, De Marco G, Di Costanzo M, Cosenza L, et al. Randomised clinical trial: efficacy of a new synbiotic formulation containing Lactobacillus paracasei B21060 plus arabinogalactan and xilooligosaccharides in children with acute diarrhoea. Aliment Pharmacol Ther. 2012;35(7):782-788.
doi pubmed - Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, Hennessy T, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32(3):331-351.
doi pubmed - Heel RC, Brogden RN, Speight TM, Avery GS. Loperamide: a review of its pharmacological properties and therapeutic efficacy in diarrhoea. Drugs. 1978;15(1):33-52.
doi pubmed - Guandalini S. Probiotics for prevention and treatment of diarrhea. J Clin Gastroenterol. 2011;45(Suppl):S149-153.
doi pubmed - Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;11:CD003048.
doi - Zhang W, Azevedo MS, Wen K, Gonzalez A, Saif LJ, Li G, Yousef AE, et al. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008;26(29-30):3655-3661.
doi pubmed - Kim A, Chang JY, Shin S, Yi H, Moon JS, Ko JS, Oh S. Epidemiology and factors related to clinical severity of acute gastroenteritis in hospitalized children after the introduction of rotavirus vaccination. J Korean Med Sci. 2017;32(3):465-474.
doi pubmed - Dinleyici EC, Eren M, Ozen M, Yargic ZA, Vandenplas Y. Effectiveness and safety of Saccharomyces boulardii for acute infectious diarrhea. Expert Opin Biol Ther. 2012;12(4):395-410.
doi pubmed - Wolvers D, Antoine JM, Myllyluoma E, Schrezenmeir J, Szajewska H, Rijkers GT. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of infections by probiotics. J Nutr. 2010;140(3):698S-712S.
doi pubmed - Fenimore A, Martin L, Lappin MR. Evaluation of metronidazole with and without enterococcus faecium SF68 in shelter dogs with diarrhea. Top Companion Anim Med. 2017;32(3):100-103.
doi pubmed - Guarino A, Lo Vecchio A, Canani RB. Probiotics as prevention and treatment for diarrhea. Curr Opin Gastroenterol. 2009;25(1):18-23.
doi pubmed - Sanders ME, Benson A, Lebeer S, Merenstein DJ, Klaenhammer TR. Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr Opin Biotechnol. 2018;49:207-216.
- Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek. 2002;82(1-4):279-289.
doi pubmed - Alam NH, Ashraf H. Treatment of infectious diarrhea in children. Paediatr Drugs. 2003;5(3):151-165.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
