| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website http://www.theijcp.org |
Case Report
Volume 9, Number 2, June 2020, pages 35-40
Food Protein Induced Enterocolitis Syndrome Presenting With Life-Threatening Methemoglobinemia: A Case Report and Review of the Literature
Aban Bahabria, f, Jasmin Moradib, Karen Choongc, Nikhil Paid, Mihir Bhatte
aDepartment of Pediatrics, McMaster Children’s Hospital, Hamilton, Ontario, Canada
bDepartment of Pediatric Critical Care, McMaster Children’s Hospital, Hamilton, Ontario, Canada
cDepartment of Pediatrics and Critical Care; Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Ontario, Canada
dDepartment of Pediatrics, Division of Pediatric Gastroenterology & Nutrition, Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, Ontario, Canada
eDepartment of Pediatrics, Division of Pediatric Hematology Oncology, McMaster University, Hamilton, Ontario, Canada
fCorresponding Author: Aban Bahabri, McMaster Children’s Hospital, 1200 Main St W, Hamilton, ON L8N 3Z5, Canada
Manuscript submitted March 23, 2020, accepted March 30, 2020, published online June 18, 2020
Short title: FPIES Presenting With Methemoglobinemia
doi: https://doi.org/10.14740/ijcp366
| Abstract | ▴Top |
Food protein induced enterocolitis syndrome (FPIES) can present with diarrhea, hypovolemia and electrolyte imbalance in infancy. We present a case of life-threatening methemoglobinemia in a 1-month-old infant, a rare complication of FPIES triggered by cow’s milk protein intake. A previously healthy 1-month-old boy presented with lethargy, increased work of breathing and 2-day history of vomiting. Review of systems revealed a 3-week history of diarrhea. He was lethargic, shocky and dusky, and was intubated for persistent hypoxia. His blood work revealed severe acidemia with pH of 6.95 and methemoglobin level of 66% (normal range < 3%). His methemoglobin level and clinical status normalized following volume resuscitation, packed red blood cell transfusion and prompt intravenous methylene blue administration. Further investigations revealed a diagnosis of FPIES which was managed with a hypoallergenic formula. Methemoglobinemia should be considered in young infants presenting with severe vomiting and diarrhea, secondary to dietary protein intolerance syndromes. Prompt management with methylene blue and fluid resuscitation can result in excellent prognosis, along with specific ongoing management for FPIES.
Keywords: Methemoglobinemia; Food protein induced enterocolitis; Cow milk protein allergy; Pediatrics
| Introduction | ▴Top |
Food protein induced enterocolitis syndrome (FPIES) is caused by dietary food intolerance, and may present with severe enterocolitis symptoms that often lead to shock [1, 2]. Cow’s milk protein allergy (CMPA) is often considered in the differential for FPIES, as it can present very similarly but with symptoms only manifested by exposure to cow’s milk. CMPA may be IgE-mediated, non-IgE-mediated or mixed in its pathophysiology. FPIES is a non-IgE, T-cell-mediated condition.
Methemoglobinemia is a functional anemia in which hemoglobin is unable to reversibly bind oxygen due to the oxidation of ferrous (Fe+2) ions of heme to the ferric (Fe+3) state. The oxygen affinity of any remaining normal ferrous heme is increased, causing left shift of the oxyhemoglobin dissociation curve and subsequent hypoxia.
| Case Report | ▴Top |
A previously healthy 1-month-old boy presented to a community hospital with lethargy, increased work of breathing and 2 days of vomiting. On initial exam, he was floppy with dusky gray skin discoloration. He had level anterior fontanel and equal reactive pupils. He was tachycardic (178 beats/min), mildly tachypneic with increased work of breathing (respiratory rate (RR) of 46) and normotensive (blood pressure (BP) 88/56 mm Hg). He was hypoxemic (oxygen saturation, SpO2 of 88%) which rose only to a maximum of 92% with 100% FiO2 administration. He was resuscitated with a total of 60 mL/kg normal saline for hypoperfusion, intubated for persistent lethargy and hypoxemia and started empirically on antibiotics for a presumed sepsis.
Initial arterial blood gas revealed a pH of 6.95, PCO2 of 17 mm Hg, PO2 of 105, bicarbonate of 4 mmol/L and hyperlactatemia of 4.7 mmol/L. His blood was noted to be dark chocolate in color and the methemoglobin (MetHb) fraction was extremely elevated at 66% (normal < 3%). Complete blood count showed white blood cell (WBC) count of 48.3 × 109/L, hemoglobin of 114 g/L, platelets of 1,190 × 109/L and C-reactive protein of 109 mg/L (1,038.12 nmol/L). Glucose and electrolytes were within normal limits. Renal and liver functions were normal: urea 2.4 mmol/L, creatinine 44 µmmol/L, aspartate aminotransferase (AST) 25 U/L, alanine aminotransferase (ALT) 50 U/L, bilirubin 6 µmmol/L and international normalized ratio (INR) of 1. Urine analysis and microscopy was negative for leukocyte esterase, nitrite, WBC, red blood cell (RBC) and protein. His lungs and cardiac silhouette were normal on the chest X-ray (Fig. 1).
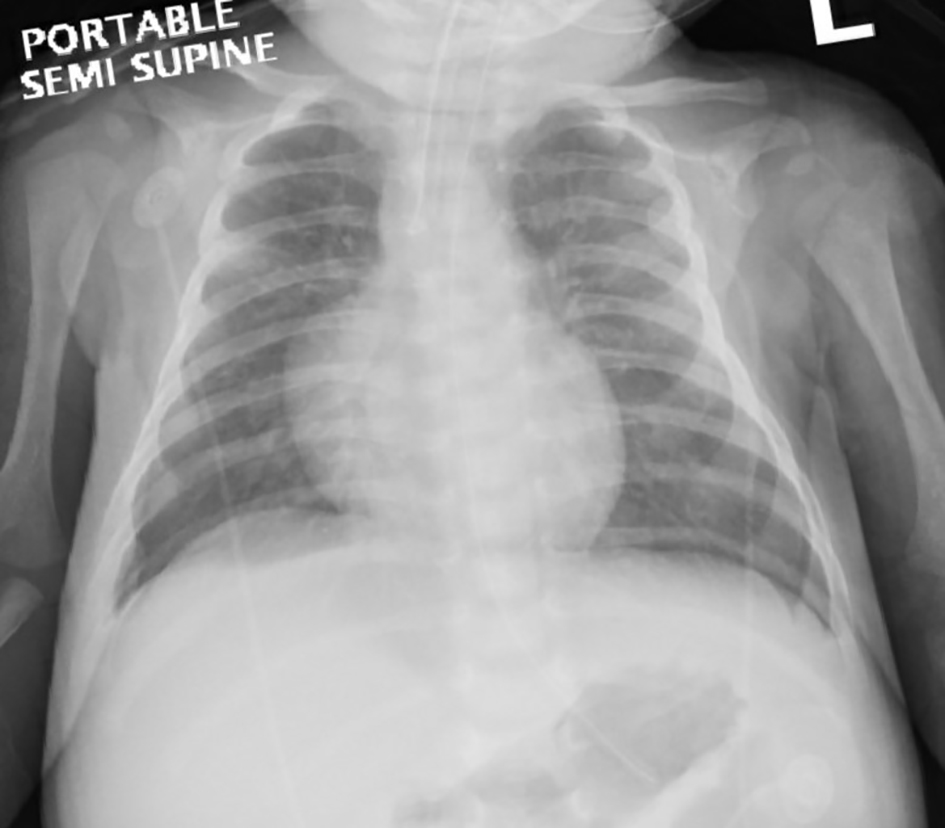 Click for large image | Figure 1. Chest X-ray after resuscitation and intubation showing normal lung fields and cardiac silhouette. |
The patient received a total of 3 mg/kg of methylene blue given in two doses 3 h apart, and packed red blood cell (pRBC) transfusion to improve oxygen delivery. He was transferred to a tertiary care pediatric intensive care unit (PICU) where preparations were made for possible exchange transfusion. On arrival to PICU 5 h after his resuscitation and administration of methylene blue, his perfusion significantly improved, and he was pink in color, sedated and intubated with an SpO2 of 98% on room air. His temperature was 36.9 °C, heart rate (HR) was 140, RR was 32 and BP was 86/49. His MetHb fraction had dropped dramatically in this period to 3%, and his lactic acidemia corrected fully (venous blood gas: pH 6.39, PCO2 38 mm Hg, PO2 78, bicarbonate 23 mmol/L). He was successfully extubated 12 h following presentation to PICU. His neurological exam after extubation was unremarkable, alert and moving all limbs and had good oral sucking. He received meningitic doses of ceftriaxone and vancomycin for 2 days until blood culture was negative. No lumbar puncture was done given the instability initially and then the rapid improvement of neurological status. Stool culture, ova and parasite were negative.
Review of systems revealed a full-term infant born at 37 weeks gestational age with normal antenatal history, Apgar scores at birth were 9 and 9 in 1 and 5 min, and birth weight was 4.337 kg. He had experienced diarrhea since his first week of life, with approximately 10 loose bowel movements per day. He was exclusively fed with a cow’s milk-based formula: initially regular formula (Enfamil A+©), then partially hydrolyzed cow’s milk-based formula (Enfamil A+ Gentlease©) 1 week prior to presentation for ongoing diarrhea. His weight was on presentation was 37 g below birth weight at 1 month of age (4.300 kg). On further history, there was no exposure to any other potential causes of methemoglobinemia such as nitrate rich well water, topical benzocaine or other medications. The infant ethnicity is mixed Jamaican and Caucasian. There was no known family history of congenital methemoglobinemia or similar presentation.
Based on this history, an initial diagnosis of severe CMPA versus FPIES was suspected and he was started on an extensively hydrolyzed hypoallergic formula. His diarrhea resolved within 6 days of initiating this formula and he continued to gain weight (Fig. 2). MetHb levels remained normal at discharge and in 3 months follow-up with hematology service. Congenital methemoglobinemia due to hemoglobin M or cytochrome B5 reductase (CYB5R) deficiency was excluded with genetic testing and normal hemoglobin electrophoresis. He continued to be followed by the pediatric gastroenterology services for FPIES management, food advancement and growth. He continued to grow well during follow-ups, but continued to experience mild diarrhea and vomiting with the introduction of other solids by 7 months of age, confirming his diagnosis of FPIES.
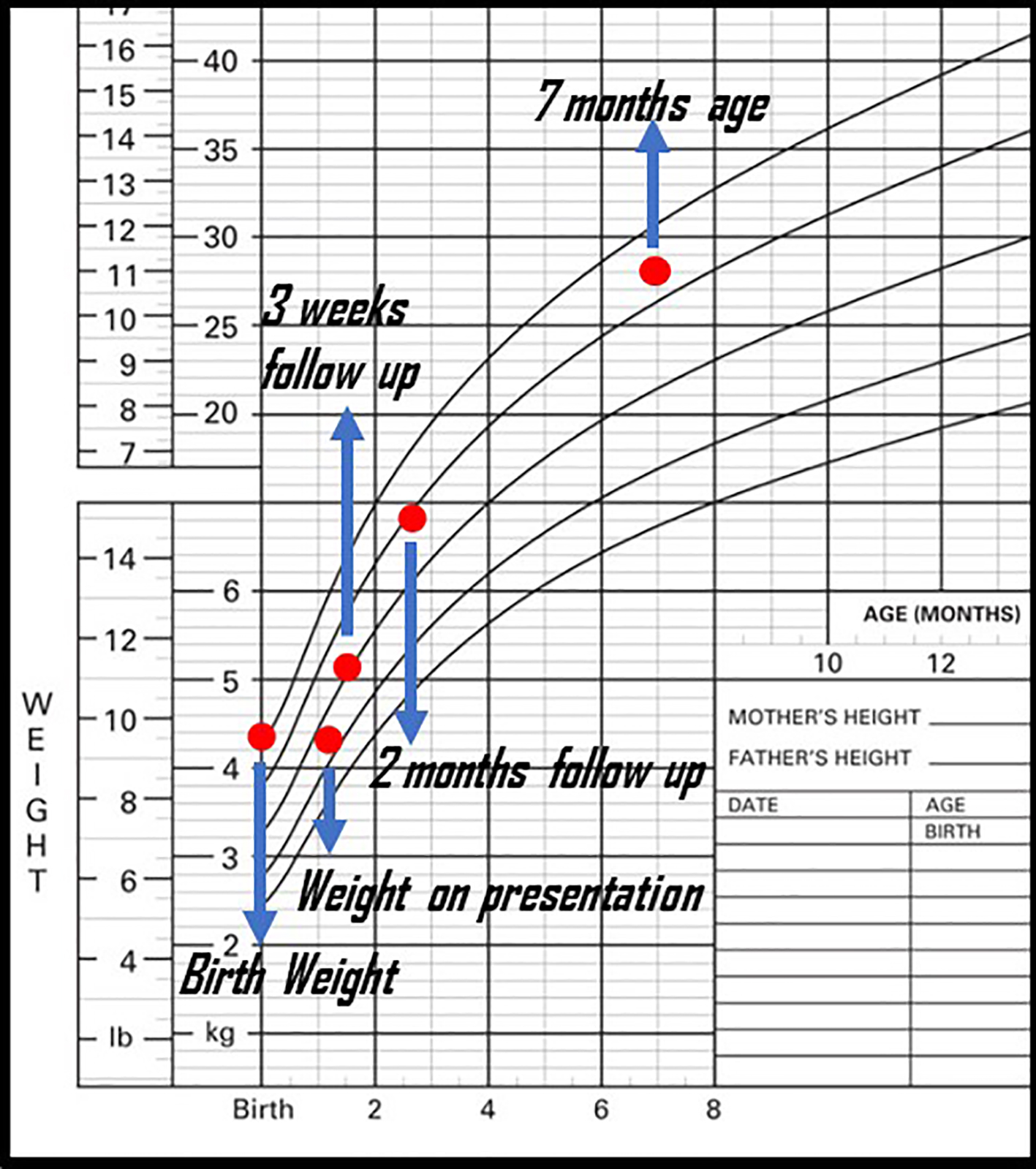 Click for large image | Figure 2. Patient’s weight gain before and after presentation. |
| Discussion | ▴Top |
This case describes the presentation of a lethal MetHb level in a patient with underlying diagnosis of FPIES, who was successfully managed with prompt methylene blue administration and fluid rehydration. This case is the highest reported MetHb level secondary to FPIES or other dietary food-protein intolerances of infancy, like CMPA. The prognosis of this child was excellent. In our case, early blood gas MetHb level led to early identification and treatment resulting in a positive outcome.
Methemoglobinemia presents with symptoms ranging from headache, cyanosis and tachycardia, to respiratory depression and shock. Symptoms often appear with levels greater than 20%; levels more than 40% are life-threatening [3]. Conventional oximeter falsely identifies MetHb as oxyhemoglobin because it has similar range of wavelength, leading to overestimating oxygen saturation [4]. Multi-wavelength CO-oximetry measures four separate wave lengths and can identify the condition. Alternatively, blood gas measuring MetHb level is another diagnostic tool.
Methemoglobinemia occurs when the ferrous (Fe+2) element of hemoglobin is oxidized to ferric (Fe+3) state. The affinity for oxygen of ferric iron is impaired and hence when methemoglobin is elevated in RBCs, tissue hypoxia occurs [5]. During hemoglobin oxygenation and deoxygenation processes and with free radical exposure, Fe+2 is converted to Fe+3, resulting in physiologic MetHb concentration [6]. Several endogenous reduction systems such as CYB5R, and to a lesser extent, NADPH-MetHb reductase through the alternative pathway, maintain the MetHb concentration below 3% in the healthy individuals (Fig. 3) [7]. CYB5R activity is low (50% of adult activity) in infants under 3 months of age, reaching adult levels by only 6 months of age [8]. Low CYB5R activity explains why young infants are at higher risk of methemoglobinemia. Methylene blue used for methemoglobinemia works by activating the alternative pathway.
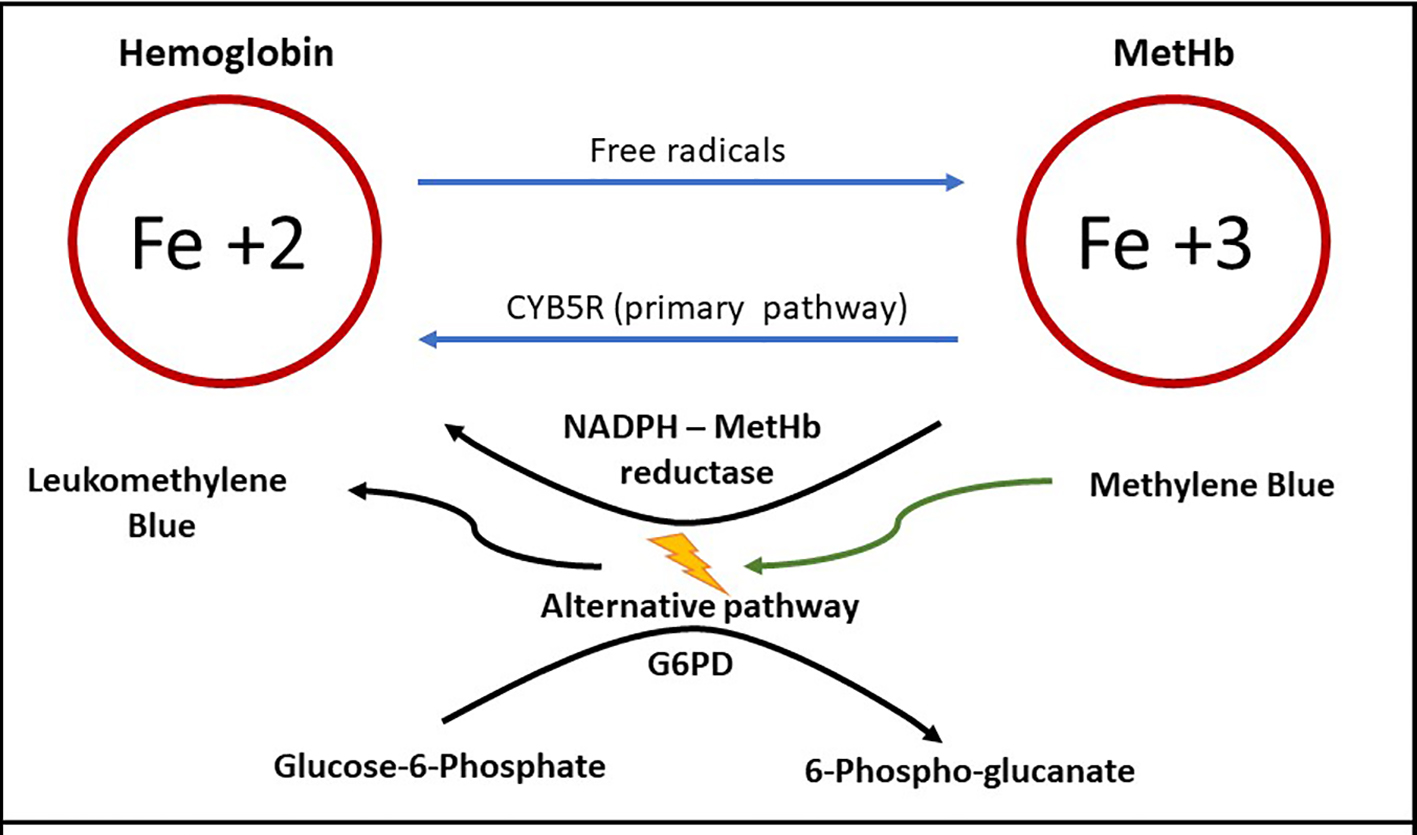 Click for large image | Figure 3. Methemoglobin (MetHb) formation, primary and alternative pathway of metabolism. Ferrous iron (Fe+2) is converted to ferric iron (Fe+3) by free radicals, forming MetHb [6]. MetHb reduced back to hemoglobin primarily by cytochrome B5 reductase (CYB5R) [7]. Activation of the alternative pathway requires methylene blue in the presence of normal levels of glucose-6-phosphate dehydrogenase (G6PD) [26]. |
Methemoglobinemia is either congenital or acquired. Congenital etiologies are very rare, and include CYB5R deficiency, cytochrome b5 deficiency or hemoglobin M. Congenital etiologies are typically asymptomatic or present with mild symptoms. Acquired methemoglobinemia is much more common, and is typically due to exposure to oxidative agents in medications or environment. Common medications include dapsone, topical anesthetic and inhaled nitric oxide. Environmental causes of methemoglobinemia are reported with vegetable or water with high nitrite level in infants, such as carrots, spinach, courgetti and well water [4, 9-11]. Other environmental exposure such as antifreeze, naphthalene (found in mothballs), nitrobenzene and alanine are also reported [12-14].
Methemoglobinemia in FPIES is due to increased systemic nitrite levels [15, 16]. Nitrites and nitrates are present in food including breast milk and infant formulas [17]. Under physiological state, the body metabolizes and excretes nitrite through gastrointestinal (GI) and renal systems (Fig. 4). Initially, intestinal bacteria reduce ingested nitrate to nitrite which is partially absorbed or subsequently converted to ammonia. Remaining nitrite is oxidized back to nitrate by colonocyte enzyme catalase or to lesser extent by erythrocyte catalase enzyme. When erythrocytes convert nitrite to nitrate, MetHb is formed but is rapidly reduced back to Hb by MetHb reductase.
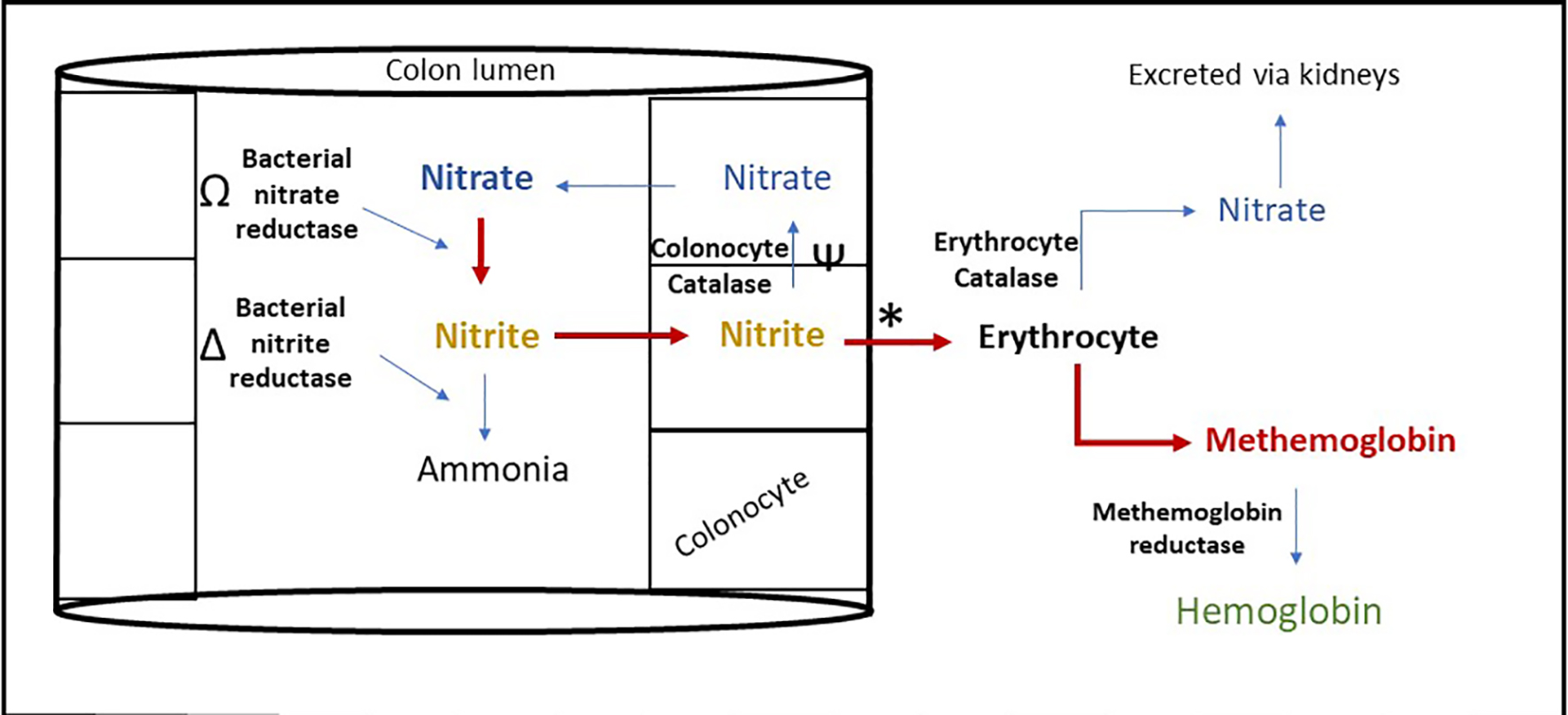 Click for large image | Figure 4. Proposed mechanism of methemoglobinemia in food protein induced enterocolitis syndrome. Nitrite metabolism is impaired in FPIES leading to increased erythrocyte nitrite metabolism inducing methemoglobinemia, through the following mechanism. Ω: Bacterial overgrowth increases the rate of this reaction [20]. Δ: Microbiome alteration alters the rate of this reaction [19]. Ψ: Mucosal catalase activity reduced in colonic inflammation [18]. *: Acidosis increases this reaction [21]. |
Increased systemic nitrite levels in FPIES are due to multiple factors (Fig. 4). With mucosal inflammation, mucosal catalase activity is reduced, and nitrite conversion depends mainly on erythrocyte catalase activity [18]. This results in decreased nitrite clearance and higher production of MetHb. Second, nitrite production and metabolism is largely regulated by intestinal microbiota. FPIES has been associated with microbiome alteration and bacterial overgrowth, which can lead to increasing nitrite production through decreased metabolism of nitrite by conventional microbial taxa [19, 20]. Third, metabolic acidemia worsens methemoglobinemia by increasing nitrite induced MetHb conversion [21]. The acidemia that accompanies FPIES is secondary to intestinal bicarbonate losses from diarrhea [22], hypovolemia, methemoglobinemia induced tissue hypoxia and resultant lactic acidemia. Infectious gastroenteritis has been reported to also cause methemoglobinemia in young infants through similar pathophysiology [23-25].
Acquired methemoglobinemia is managed by discontinuing the offending agent and using methylene blue. Methylene blue works by activating the alternative MetHb reduction pathway (Fig. 3) [26]. Methylene blue is given at a usual dose of 1 - 2 mg/kg as first line treatment. Typically, a single dose is efficient and results in rapid response in less than 1 h [27]. As demonstrated in Figure 3, methylene blue activates the alternative pathway which requires G6PD enzyme. For this reason, it is contraindicated in patients who have or suspected to have G6PD as it induces hemolysis, ineffective and may even worsen the methemoglobinemia [28]. If methylene blue is not available, ineffective or contraindicated, high dose intravenous ascorbic acid (vitamin C) may be used in a patient [29]. This effect of vitamin C is thought to be due to its antioxidant effect, which reduces MetHb production. Intravenous vitamin C should only be used in a patient with normal renal function as high dose ascorbic acid can cause oxalate nephropathy.
We conducted a literature review and summarized the 32 reported methemoglobinemia cases associated with FPIES or CMPA (Table 1) [18, 23, 30-35]. There are key similarities to these cases, all having occurred in infants under 3 months of age, and the duration from diarrhea to presentation ranging from 2 days to 2 weeks. There is no correlation between the level of MetHb and acidosis severity. The absence of this correlation may be explained by the patient’s baseline CYB5R enzyme level; higher enzyme levels result in lower MetHb levels. Conversely, other factors such as volume status and the degree of intestinal bicarbonate losses contribute to greater acidosis, further lowering MetHb levels.
 Click to view | Table 1. Summarized Case Reports of Methemoglobinemia Associated With FPIES or CMPA |
In summary, this case demonstrates the role of FPIES, and other dietary protein intolerance conditions in causing severe, life-threatening methemoglobinemia and metabolic acidosis. It is prudent to consider FPIES in infants presenting with a shock-like picture, especially with a history of diarrheal disease. In those situations, sending a MetHb level is critical to the early detection and treatment of methemoglobinemia.
Acknowledgments
We would like to thank the family who agreed on sharing their child’s story.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
Dr. Aban Bahabri contributed to writing the case, discussion and review of the literature. Dr. Jasmine Moradi contributed to writing the case and helping in edits. Dr. Karen Choong contributed to helping in edits from ICU perspective. Dr. Nikhil Pai contributed to helping in edits from GI perspective. Dr. Mihir Bhatt contributed to helping in edits from hematology perspective.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Nowak-Wegrzyn A, Sampson HA, Wood RA, Sicherer SH. Food protein-induced enterocolitis syndrome caused by solid food proteins. Pediatrics. 2003;111(4 Pt 1):829-835.
doi pubmed - Caubet JC, Bencharitiwong R, Ross A, Sampson HA, Berin MC, Nowak-Wegrzyn A. Humoral and cellular responses to casein in patients with food protein-induced enterocolitis to cow's milk. J Allergy Clin Immunol. 2017;139(2):572-583.
doi pubmed - Rehman HU. Methemoglobinemia. West J Med. 2001;175(3):193-196.
doi pubmed - Keating JP, Lell ME, Strauss AW, Zarkowsky H, Smith GE. Infantile methemoglobinemia caused by carrot juice. N Engl J Med. 1973;288(16):824-826.
doi pubmed - Darling R, Roughton F. The effect of methemoglobin on the equilibrium between oxygen and hemoglobin. Am J Physiol. 1942;137:56.
doi - Mansouri A, Lurie AA. Concise review: methemoglobinemia. Am J Hematol. 1993;42(1):7-12.
doi pubmed - Eder HA, Finch C, McKee RW. Congenital Methemoglobinemia. A Clinical and Biochemical Study of a Case. J Clin Invest. 1949;28(2):265-272.
doi pubmed - Nilsson A, Engberg G, Henneberg S, Danielson K, De Verdier CH. Inverse relationship between age-dependent erythrocyte activity of methaemoglobin reductase and prilocaine-induced methaemoglobinaemia during infancy. Br J Anaesth. 1990;64(1):72-76.
doi pubmed - Sander C, Jacobi H. [Methemoglobin poisoning in a 2-year old boy after eating spinach]. Z Kinderheilkd. 1967;98(3):222-226.
doi pubmed - Hack WW, Douwes AC, Veerman AJ. [Spinach: A source of nitrite poisoning in young children]. Ned Tijdschr Geneeskd. 1983;127(32):1428-1431.
- Savino F, Maccario S, Guidi C, Castagno E, Farinasso D, Cresi F, Silvestro L, et al. Methemoglobinemia caused by the ingestion of courgette soup given in order to resolve constipation in two formula-fed infants. Ann Nutr Metab. 2006;50(4):368-371.
doi pubmed - Lee CH, Kim SH, Kwon DH, Jang KH, Chung YH, Moon JD. Two cases of methemoglobinemia induced by the exposure to nitrobenzene and aniline. Ann Occup Environ Med. 2013;25(1):31.
doi pubmed - Sohn CH, Seo DW, Ryoo SM, Lee JH, Kim WY, Lim KS, Oh BJ. Life-threatening methemoglobinemia after unintentional ingestion of antifreeze admixtures containing sodium nitrite in the construction sites. Clin Toxicol (Phila). 2014;52(1):44-47.
doi pubmed - Volney G, Tatusov M, Yen AC, Karamyan N. Naphthalene Toxicity: Methemoglobinemia and Acute Intravascular Hemolysis. Cureus. 2018;10(8):e3147.
doi - Hegesh E, Shiloah J. Blood nitrates and infantile methemoglobinemia. Clin Chim Acta. 1982;125(2):107-115.
doi - Roediger WE, Lawson MJ, Nance SH, Radcliffe BC. Detectable colonic nitrite levels in inflammatory bowel disease - mucosal or bacterial malfunction? Digestion. 1986;35(4):199-204.
doi pubmed - Jones JA, Ninnis JR, Hopper AO, Ibrahim Y, Merritt TA, Wan KW, Power GG, et al. Nitrite and nitrate concentrations and metabolism in breast milk, infant formula, and parenteral nutrition. JPEN J Parenter Enteral Nutr. 2014;38(7):856-866.
doi pubmed - Murray KF, Christie DL. Dietary protein intolerance in infants with transient methemoglobinemia and diarrhea. J Pediatr. 1993;122(1):90-92.
doi - Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, Morris A. Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med. 2017;105:48-67.
doi pubmed - Stockbrugger RW, Cotton PB, Eugenides N, Bartholomew BA, Hill MJ, Walters CL. Intragastric nitrites, nitrosamines, and bacterial overgrowth during cimetidine treatment. Gut. 1982;23(12):1048-1054.
doi pubmed - Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin: a mechanistic study. J Biol Chem. 2008;283(15):9615-9622.
doi pubmed - Peduto A, Rocca M, De Maio C, Gallarotti F, Pomero G, Gancia P. Metabolic acidosis as Food Protein Induced Enterocolitis Syndrome (FPIES) onset in a newborn. Ital J Pediatr. 2018;44(1):52.
doi pubmed - Hanukoglu A, Danon PN. Endogenous methemoglobinemia associated with diarrheal disease in infancy. J Pediatr Gastroenterol Nutr. 1996;23(1):1-7.
doi pubmed - Babbitt CJ, Garrett JS. Diarrhea and methemoglobinemia in an infant. Pediatr Emerg Care. 2000;16(6):416-417.
doi pubmed - Gebara BM, Goetting MG. Life-threatening methemoglobinemia in infants with diarrhea and acidosis. Clin Pediatr (Phila). 1994;33(6):370-373.
doi pubmed - Beutler E, Baluda MC. Methemoglobin Reduction. Studies of the Interaction between Cell Populations and of the Role of Methylene Blue. Blood. 1963;22:323-333.
doi pubmed - Kane GC, Hoehn SM, Behrenbeck TR, Mulvagh SL. Benzocaine-induced methemoglobinemia based on the Mayo Clinic experience from 28 478 transesophageal echocardiograms: incidence, outcomes, and predisposing factors. Arch Intern Med. 2007;167(18):1977-1982.
doi pubmed - Sikka P, Bindra VK, Kapoor S, Jain V, Saxena KK. Blue cures blue but be cautious. J Pharm Bioallied Sci. 2011;3(4):543-545.
doi pubmed - Lee KW, Park SY. High-dose vitamin C as treatment of methemoglobinemia. Am J Emerg Med. 2014;32(8):936.
doi pubmed - Malin SW, Lutfi R, Friedman ML, Teagarden AM. Food protein-induced enterocolitis syndrome causing hypovolemic shock and methemoglobinemia. Case Rep Crit Care. 2018;2018:1903787.
doi pubmed - Anand RK, Appachi E. Case report of methemoglobinemia in two patients with food protein-induced enterocolitis. Clin Pediatr (Phila). 2006;45(7):679-682.
doi pubmed - Venkatesh HA. Cow's milk -an unusual cause of methemoglobinemia in an infant. Int J Life Sci Res. 2014;2(3):136-137.
- Rishi M, Chaudhry S. Case 1: A blue infant with chocolate-coloured blood. Paediatr Child Health. 2011;16(6):333-334.
doi pubmed - Yano SS, Danish EH, Hsia YE. Transient methemoglobinemia with acidosis in infants. J Pediatr. 1982;100(3):415-418.
doi - Khodayar-Pardo P. Methemoglobinemia: key in the diagnosis of food protein-induced enterocolitis syndrome by cow's milk protein in infants. EC Gastroenterol Dig Syst. 2016;1.4:129-132.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
