| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Case Report
Volume 11, Number 1, March 2022, pages 9-13
Subcutaneous Tissue Swelling and Prolonged Edema: Unexpected Outcomes of the Disinfection Through Octenidine Dihydrochloride (Octenisept®)
Odorizzi Marcoa, b, Flurim Hamitagaa, Allasia Alessiaa
aDepartment of Orthopaedic Pediatric Surgery, San Giovanni Hospital, Bellinzona, Switzerland
bCorresponding Author: Odorizzi Marco, Department of Orthopaedic Pediatric Surgery, San Giovanni Hospital, Bellinzona, Switzerland
Manuscript submitted November 1, 2021, accepted February 8, 2022, published online March 29, 2022
Short title: Subcutaneous Tissue Swelling and Prolonged Edema
doi: https://doi.org/10.14740/ijcp437
| Abstract | ▴Top |
Wound management in the pediatric age commonly deals with penetrating injuries, involving a range of structures, such as skin, blood vessels, bones, nerves, tendons and joints. Octenidine dihydrochloride (Octenisept®) is an antiseptic agent for disinfection of acute or chronic wounds. Its broad spectrum efficacy has been a key factor to spread its utilization in many outpatient clinics. This paper reports on the outcomes of the use of Octenisept® to treat prolonged edema and tissue swelling of deep wounds in three children, aged between 3 and 8 years old. The underlying physiopathological mechanism remains unclear. All patients presented a full recovery of the problem at 6 months of the incident and no late complications or disabilities were associated. We recommend not irrigating octenidine dihydrochloride in any complex or penetrating wounds.
Keywords: Subcutaneous tissue swelling; Edema; Octenidine dihydrochloride
| Introduction | ▴Top |
Prevention and treatment of tissue infection are routine procedures for wound management in pediatric surgery. Several aseptic agents are available in the market and all have been evaluated for their efficacy and side effects, including for being non-irritating, antimicrobial and for not suppressing the physiological healing processes. Among these substances, Octenisept® (octenidine dihydrochloride/phenoxyethanol solution) has become one of the most used antiseptic agents in many pediatric outpatient clinics due to its broad spectrum of efficacy, its easiness to apply, its timely full effect and its residual effects. According to the manufacturer, its use ranges from the treatment of wounds in the skin and mucosa, pre-operatory disinfection of mucous membranes and skin, and supportive handling of healing wounds. Conversely, Octenisept® is not recommended for rinsing the abdominal cavity (e.g., intraoperatively), the urinary bladder and the nose or the eardrum [1]. However, a limited literature is available on its effectiveness and risks in all the spectrum of wounds. In this paper, we report three cases of chronic inflammation in pediatric patients due to severe tissue necrosis after irrigation of penetrating wounds with Octenisept®.
| Case Reports | ▴Top |
Case 1
A 3-year-old boy, in regular good health, without medical or surgery history, presented a III grade burn in his forearm and was subjected to Thiersch graft head (scalp). By accident, during the graft surgery, octenidine dihydrochloride (about 80 mL) was used to infiltrate the scalp donor site of the head instead of NaCl 0.9%. Postoperatively, a significant and persistent head edema and loosing of hair was recorded.
An ultrasound showed the presence of a hypoechogenic structure located in between the external tabula and the subcutis. The blood test noted a mild inflammatory reaction (C-reactive protein (CRP) 63 mg/L, Hb 10.4 g/dL, leukocyte 11.6 g/L, and thrombocyte 444 g/L).
The examinations did not show collections and the clinical aspect suggested a toxic-allergic reaction of undetermined origin. However, the appearance of fever peaks did not allow excluding an infectious origin. In agreement with the pediatric infectiologist onsite, an antibiotic intravenous therapy (amoxicillin 50 mg/kg/8 h) was started with gradual local improvements.
Due to the persistence of the edema for 1 month and no changes in the outcomes of a further magnetic resonance imaging (MRI), a drainage procedure was performed with the removal of only serous and non-purulent fluid. The microbiological investigation of the wound smear did not reveal any significant results. It was decided to introduce prednisolone on a sliding scale and the patient was regularly monitored in our outpatient clinic: swelling and induration improved progressively and the hair restarted to grow after 4 months. Complete resolution of the problem was attained 6 months after the incident.
Case 2
A 7-year-old boy with a wound on his right hand secondary to a dog’s bite arrived in our institution. No medical history was registered. The wound was cleaned and irrigated with octenidine dihydrochloride (about 60 mL) and NaCl 0.9%, treated with oral antibiotic (co-amoxicillin 25 mg/kg/12 h) and immobilized with a splint cast. Despite of the treatment, the hand continued to be swollen in the following days and became hyperemic and indurated with a consequent functional limitation (Fig. 1).
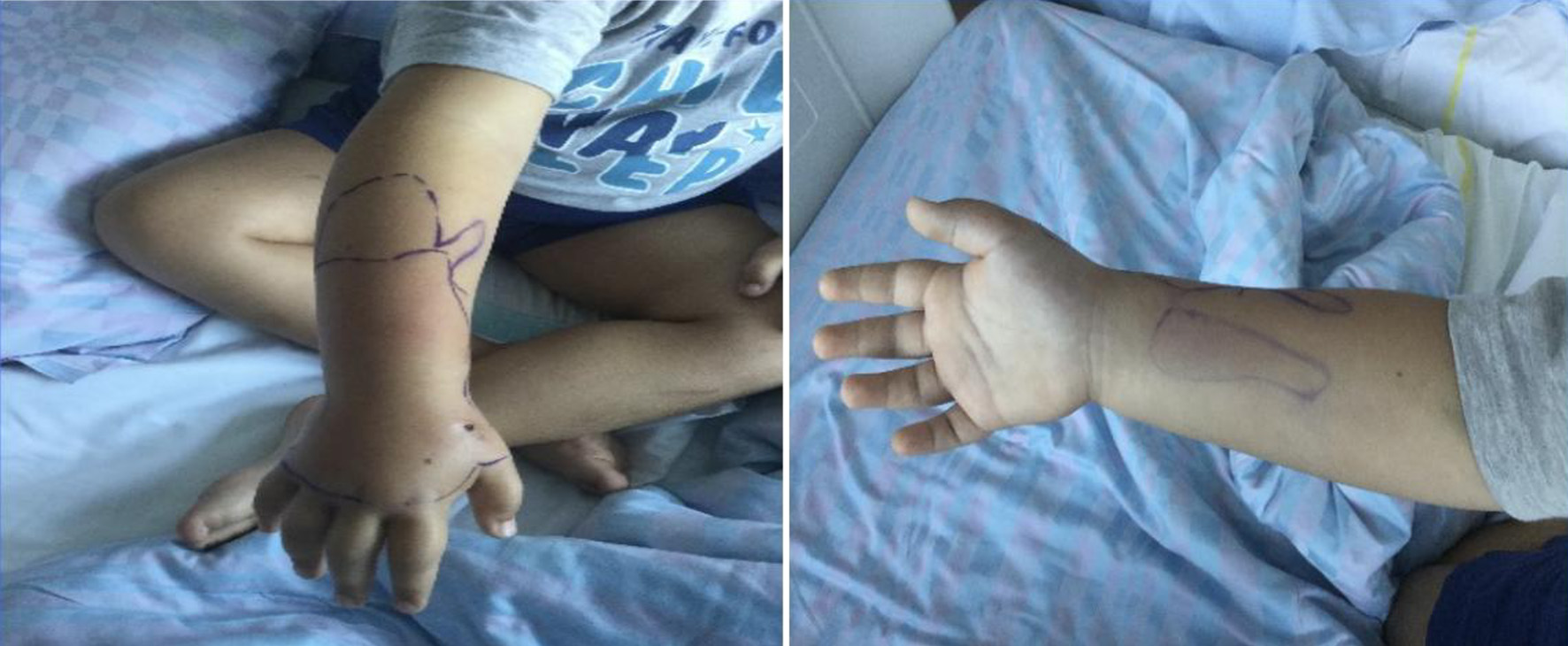 Click for large image | Figure 1. Clinical presentation after 72 h. |
No other symptoms were observed. An intravenous antibiotics treatment (co-amoxicillin 50 mg/kg/8 h) was started and an orthopedic gilet was placed. After more than 72 h of antibiotic therapy, a worsening of the local edema, associated with a slight rise of inflammatory index (CRP to 32 mg/L; leukocyte 7.1 g/L), was detected. In agreement with the pediatric infectiologist onsite, the antibiotic therapy was replaced with piperacillin-tazobactam (3 g/8 h) and an anti-inflammatory (ibuprofen 10 mg/kg/8 h) was started.
An ultrasound showed a massive soft tissue edema with a cellulite-surrounded area, without evidence of abscess or remaining foreign material (Fig. 2).
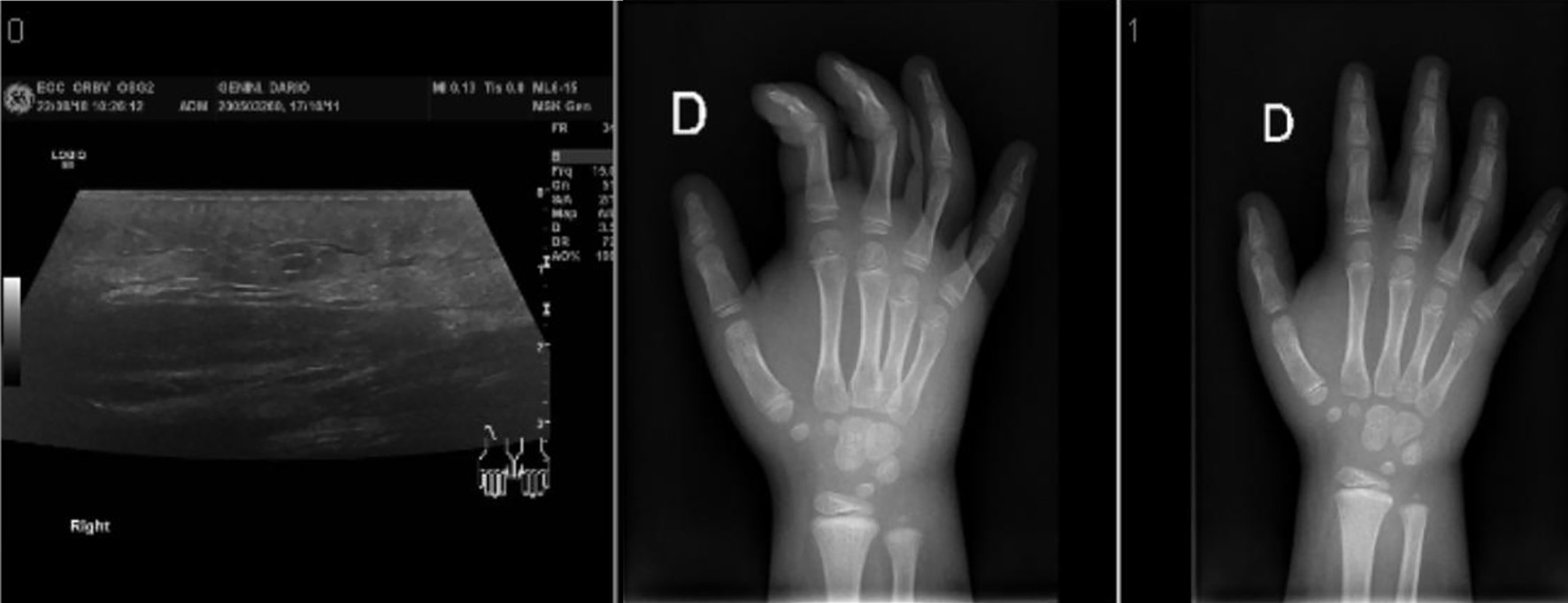 Click for large image | Figure 2. Ultrasound and X-ray imaging showing the soft tissue edema. |
The microbiological investigation of the wound smear did not reveal any significant results. The blood test did not provide any substantial evidence of inflammation parameters and the patient showed no fever during the period.
The patient was regularly examined in our outpatient clinic after the first visit: swelling and induration started to decline after 4 months until a complete resolution in 6 months (Fig. 3).
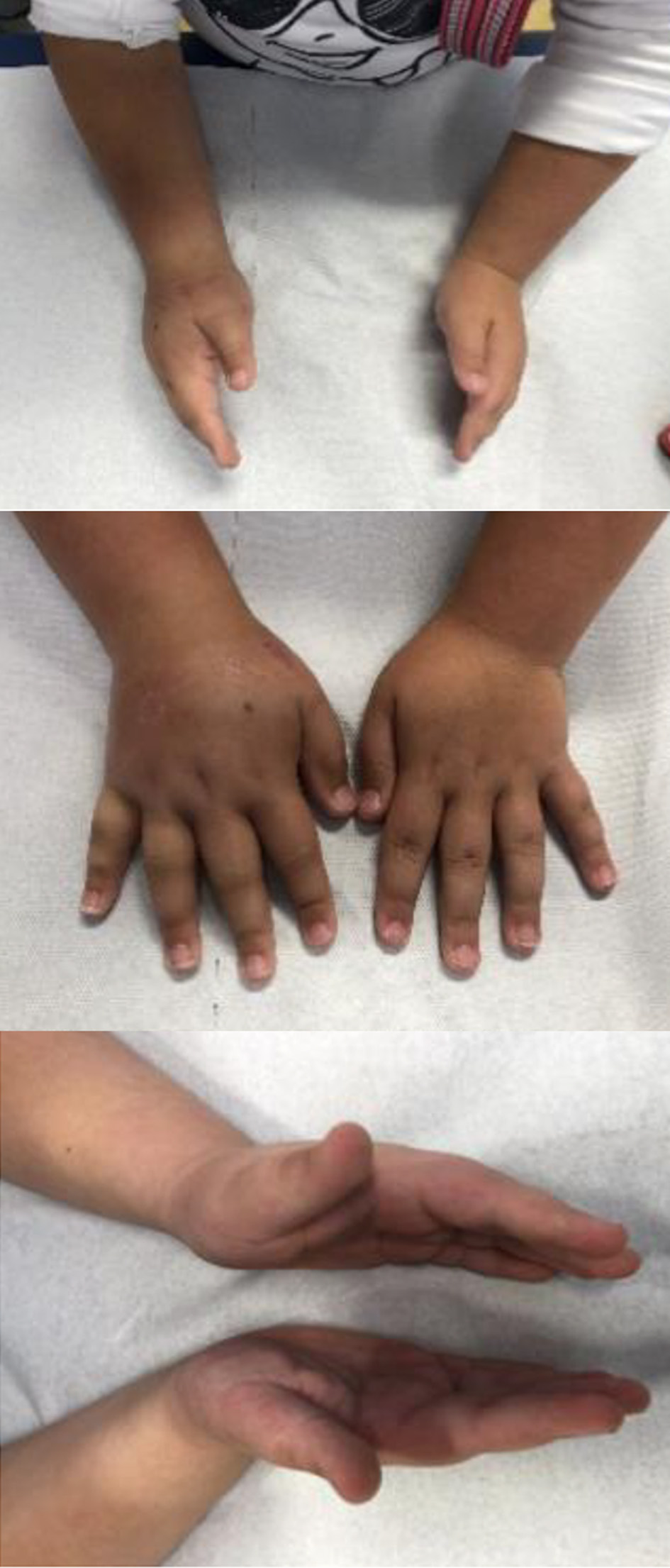 Click for large image | Figure 3. Follow-up after 6 months. |
Case 3
An 8-year-old boy, without any medical history, arrived in our institution with a plantar foot wound caused by a non-rusted nail. The wound was cleaned and irrigated with octenidine dihydrochloride (about 60 mL) and NaCl 0.9%, treated with oral antibiotic (co-amoxicillin 25 mg/kg/12 h) and the foot was immobilized with a splint cast. Due to the persistence of swelling and functional limitation correlated with a rise of the inflammation index (white blood cells: 11,400/mm3; CRP 42 mg/L), the patient was hospitalized and an intravenous antibiotics treatment (co-amoxicillin 50 mg/kg/8 h) was introduced. During the hospitalization, the patient remained apyretic with stability of the cellulite area. Mild pain and local flushing were registered. An ultrasound and an MRI reported important myositis and plantar fasciitis without abscess or fluid collections (Fig. 4).
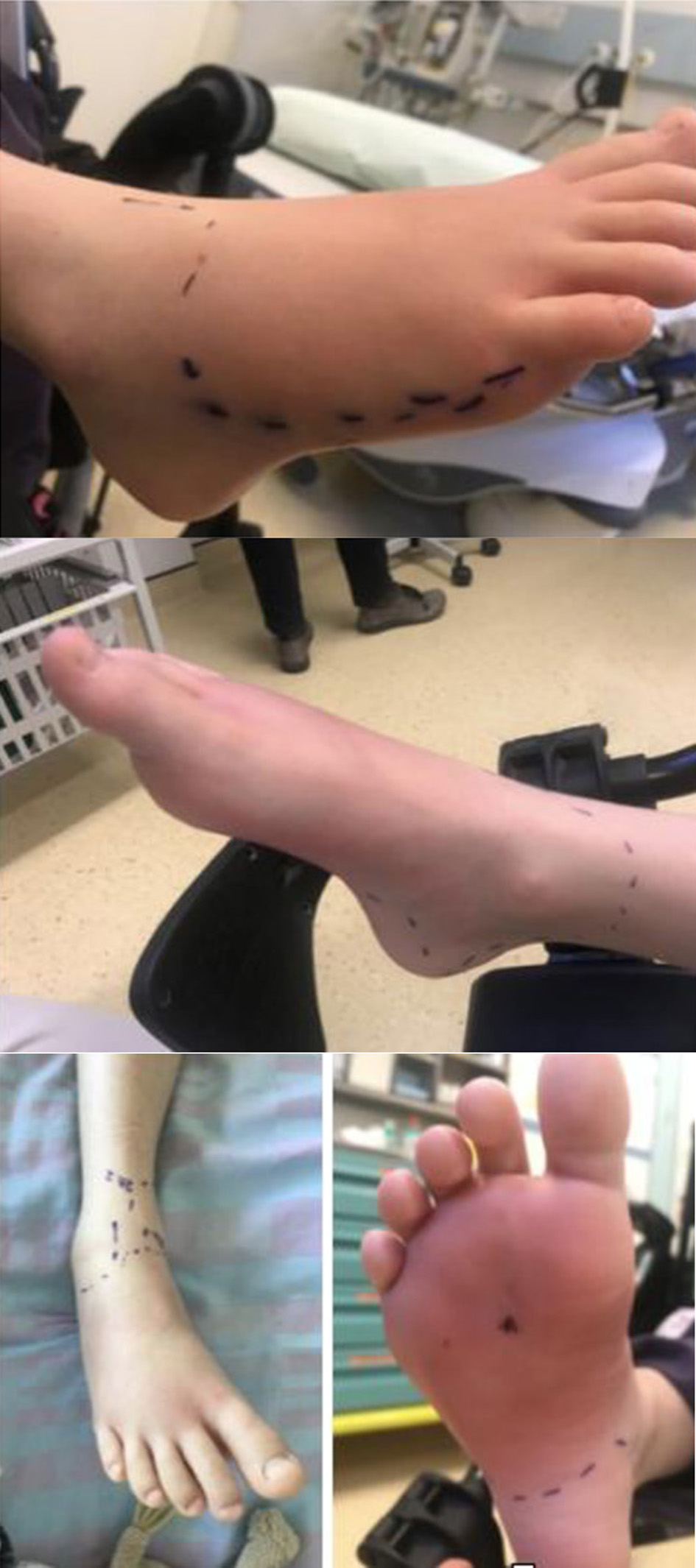 Click for large image | Figure 4. Clinical presentation after 48 h. |
In agreement with the pediatric infectiologist onsite, the antibiotic therapy was swapped into piperacillin-tazobactam (3 g/8 h) and an anti-inflammatory (ibuprofen 10 mg/kg/8 h) was started.
The microbiological investigation of the wound smear did not reveal any significant results. The blood test did not provide any substantial evidence of any inflammation parameters and the patient showed no fever during the period.
The patient was regularly examined in our outpatient clinic after the first visit and a swelling and induration remission was observed over a period of 4 months (Fig. 5).
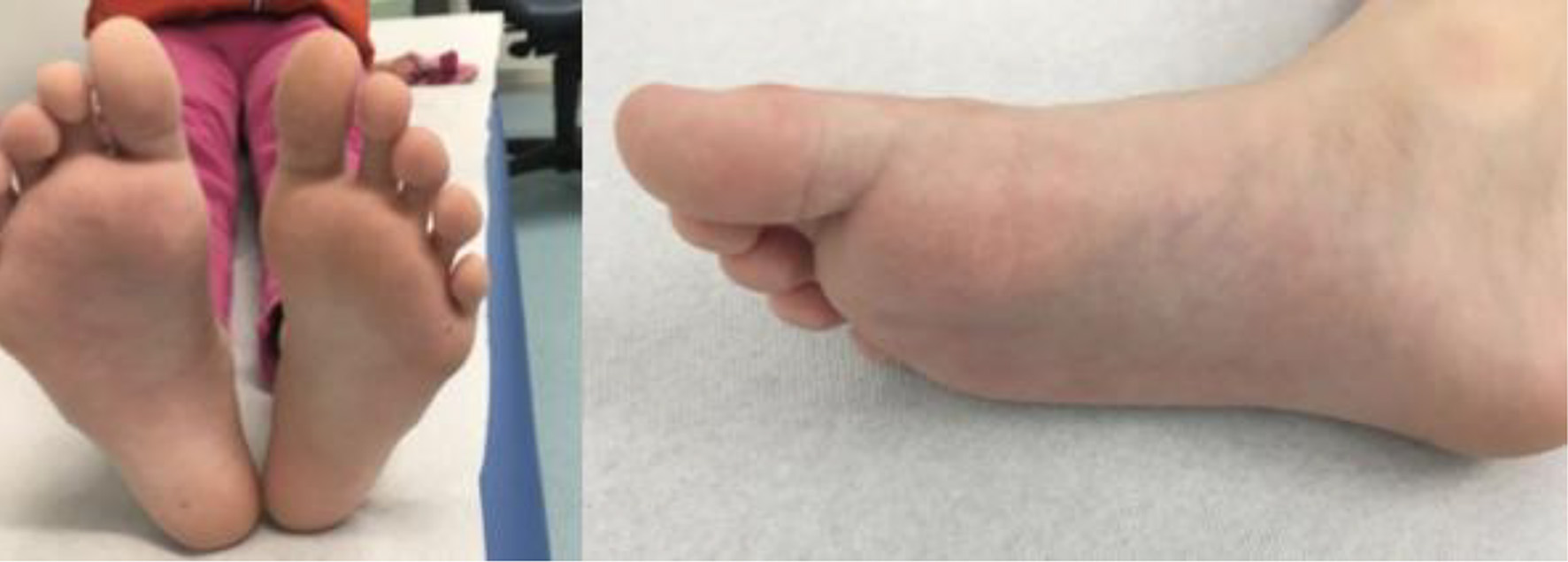 Click for large image | Figure 5. Follow-up after 4 months. |
| Discussion | ▴Top |
Octenisept® has been intentionally used to disinfect two wounds potentially contaminated and accidentally used in one surgical procedure.
We observed aseptic subcutaneous tissue swelling and edema in all three cases after the wound was lavaged and irrigated with Octenisept®. This occurred shortly after the wounds were initially treated and lasted for at least 16 weeks until the symptoms slowly declined.
No other symptoms were registered. All patients received intravenous antibiotic treatment in the first days. All serial ultrasounds performed to control the inflammatory reaction showed massive soft tissue edema with surrounded cellulite and excluded evidence of abscess or remaining foreign material. The microbiological investigations of the wound smears did not show bacterial growing in any case. The blood test did not show evidence of important rising of the inflammatory parameters and all patients remained without fever during the period. All patients presented a full recovery of the problem at 6 months of the incident and no late complications or disabilities were associated.
We observed in all three patients a similar clinical outcome after the irrigation of Octenisept®: a marked indurated edematous swelling, albeit not associated with a persistent general infection, such as an increase in laboratory markers (e.g., CRP or leukocytes) or fever. Pain was registered in only one case.
No significant outcomes were registered from the microbiological investigation of the wound smear. In all the cases, radiological investigations - ultrasound and MRI - reported a massive soft tissue edema and inflammation, with no evidence of abscess, fluid collections or foreign material.
All of the patients underwent a conservative management and strict follow-up examinations reported a slow decline in the swelling, with a complete resolution within 4 to 6 months.
Although all patients presented a full recovery of the problem and no late complications or disabilities were associated, the underlying physiopathological mechanism remains unclear.
Langer et al showed that Octenisept® increases the diameter of blood vessels without causing functional impairment in the intact skin of mice [2]. The marked edema seems to be rooted into an increased vascular permeability, also observed through in-vitro testing of different topical antiseptic solutions. Octenidine dihydrochloride generates a chemical reaction with the cell membrane of micro-organisms and destroys the cell [3]. Moreover, Octenisept® significantly reduced adipose-derived stem cell viability, proliferation, and differentiation and caused considerable autoimmune sclerosing cholangitis (ASC) irreversible necrosis and therefore more detrimental form of cell death. Such necrosis is in contrast to apoptosis, which is a well-controlled form of cell death that may be inhibited by certain measures, and is followed by an inflammation, which may further prolong the critical condition of wound repair disorders. Octenisept® also reduced the expression of the stem cell markers CD29, CD34, CD90, and CD105 [4].
Wound antiseptics are cytotoxic and may also react with wound dressings. It is important to be mindful of the efficacy and the possible cytotoxicity of antiseptics on human skin and wounds; however, well-spread information about cytotoxicity of common skin antiseptics is still lacking and further studies are necessary to elucidate their physiopathological repercussion [5-7].
In the medical literature, not many publications can be found about clinical effects in deep or complex wounds treated with Octenisept®. Hulsemann et al reported similar findings in five children with severe swelling and induration after Octenisept® irrigation of penetrating hand wounds. Contaminated wounds were operated and debrided. Long-term complications characterized by scars and contractures due to fibrosis of the muscle have been observed [8].
Schupp and Holland-Cunz reported three further patients that developed subcutaneous tissue swelling and interstitial edema after initial wound lavage of the cheek or the gluteal area with Octenisept®. A drainage was performed in one of the patients [9].
A special warning label was added by the Pharmaceutic Company of the product in the information sheet/description of Octenisept® in 2004, after the notification of the first side effect: “Do not swallow Octenisept® and do not allow Octenisept® to pass into the circulation, e.g. as a result of accidental injection. When irrigating wounds, ensure that the preparation is not irrigated under pressure or injected into the tissue. When irrigating wound cavities, ensure that there is always a drainage point (e.g. drain, tab).”
An additional warning was issued in February 2008 by Schulke and Mayr concerning some complications of edematous tissue swelling after lavage of stab wounds of the hands with Octenisept®. Octenisept® was applied in all these incidents under high pressure, with the agent not sufficiently drained. The swelling was significant and accompanied at times by subcutaneous tissue necrosis. This outcome was reported to last from several weeks to months in some patients, mostly after a surgical intervention. Schulke and Mayr presumed that the remaining Octenisept® was not absorbed sufficiently and generated the reported side effects, as the symptoms were indistinguishable to those caused by the accidental injection of Octenisept® into subcutaneous tissue or muscles. Schulke and Mayr concluded that Octenisept® is not recommended for applications in wound cavities under high pressure and that adequate drainage is obligatory [10].
In the same year, the manufacturer of Octenisept® (Schulke&Mayr, Ltd) issued an additional boxed warning to the consumer and professional information sheets of this antiseptic. As the underlying mechanism of this chronic inflammatory response is not fully clarified, further studies are necessary to elucidate the physiopathological mechanisms of these side effects of Octenisept®.
Conclusion
Wound lavage and irrigation with octenidine dihydrochloride (Octenisept®) in three patients showed an aseptic, painful subcutaneous tissue swelling and edema. The underlying physiopathological mechanism triggers further investigations. Hence, we recommend not using octenidine dihydrochloride for irrigation of any complex and deep wounds. Moreover, aseptic fatty tissue necrosis and edema were observed after using Octenisept® and resolution took up to 6 months to complete. Further antibiotic treatment or surgical intervention did not prove any advantages.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consents have been obtained.
Author Contributions
Marco Odorizzi: primary author, conceptualization and research, drafting and review. Flurim Hamitaga: assistant author, reviewer. Alessia Allasia: co-primary author, conceptualization and research, drafting and review.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Schulke&Mayr. Octenisept®. http://www.schuelke-mayr.com.
- Langer S, Sedigh Salakdeh M, Goertz O, Steinau HU, Steinstraesser L, Homann HH. The impact of topical antiseptics on skin microcirculation. Eur J Med Res. 2004;9(9):449-454.
- Kalteis T, Luring C, Schaumburger J, Perlick L, Bathis H, Grifka J. [Tissue toxicity of antiseptics]. Z Orthop Ihre Grenzgeb. 2003;141(2):233-238.
doi pubmed - Kim BS, Ott V, Boecker AH, Stromps JP, Paul NE, Alharbi Z, Cakmak E, et al. The effect of antiseptics on adipose-derived stem cells. Plast Reconstr Surg. 2017;139(3):625-637.
doi pubmed - Hirsch T, Koerber A, Jacobsen F, Dissemond J, Steinau HU, Gatermann S, Al-Benna S, et al. Evaluation of toxic side effects of clinically used skin antiseptics in vitro. J Surg Res. 2010;164(2):344-350.
doi pubmed - Marquardt C, Matuschek E, Bolke E, Gerber PA, Peiper M, J VS-K, Buhren BA, et al. Evaluation of the tissue toxicity of antiseptics by the hen's egg test on the chorioallantoic membrane (HETCAM). Eur J Med Res. 2010;15(5):204-209.
doi pubmed - Muller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61(6):1281-1287.
doi pubmed - Hulsemann W, Habenicht R. [Severe side effects after Octenisept irrigation of penetrating wounds in children]. Handchir Mikrochir Plast Chir. 2009;41(5):277-282.
doi pubmed - Schupp CJ, Holland-Cunz S. Persistent subcutaneous oedema and aseptic fatty tissue necrosis after using octenisept. Eur J Pediatr Surg. 2009;19(3):179-183.
doi pubmed - Schulke Mayr. Wichtige Information zur Arzneimittelsicherheit vonOctenisept ® (Octenidindihydrochlorid, Phenoxyethanol) O demat o seSchwellungen und Gewebesch a digungen nach Einbringen unter Druckin Stichwunden bei handchirurgischen Eingriff en. Boxed warning, Norderstedt 07. February 2008.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
