| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Original Article
Volume 11, Number 3, October 2022, pages 76-84
Reduction of Exercise Capacity, Respiratory and Peripheral Muscle Strength in Severe Asthma
Natalia Marcolina, e, Bruna Barcellosa, Helena Teresinha Mocelinb, c, Gilberto Bueno Fischerb, d, Janice Luisa Lukrafkaa
aDepartment of Physiotherapy, Universidade Federal de Ciencias da Saude de Porto Alegre, Porto Alegre, Brazil
bDepartment of Pediatrics, Universidade Federal de Ciencias da Saude de Porto Alegre, Porto Alegre, Brazil
cDepartment of Pediatrics, Hospital Materno Infantil Presidente Vargas, Porto Alegre, Brazil
dDepartment of Pediatrics, Hospital da Crianca Santo Antonio, Porto Alegre, Brazil
eCorresponding Author: Natalia Marcolin, Pediatrics, Universidade Federal de Ciencias da Saude de Porto Alegre, Porto Alegre, Brazil
Manuscript submitted January 22, 2022, accepted October 14, 2022, published online October 31, 2022
Short title: Exercise Capacity in Severe Asthma
doi: https://doi.org/10.14740/ijcp477
| Abstract | ▴Top |
Background: Patients with severe asthma need to be studied separately due to differences from less severe asthma. This is the first study to carry out a wide physical evaluation of children and adolescents with severe asthma. The objective was to evaluate maximal and submaximal exercise capacity, respiratory and peripheral muscle strength in children and adolescents with severe asthma.
Methods: The study included children and adolescents (6 to 18 years) diagnosed with severe asthma, controlled in the last 4 weeks. The maximal exercise capacity was measured by the modified shuttle test (MST) and submaximal by the 6-min walk test. Respiratory muscle strength was evaluated by measuring maximal respiratory pressures and the peripheral strength through a handgrip.
Results: Thirty patients were included, with mean age of 10.3 years, 63% female and 46.7% overweight or obese. Patients underperformed but reached more than 80% of expected in all evaluations. There was a moderate correlation between age and body mass index (BMI) with MST, maximal expiratory pressure and peripheral muscle strength. The correlation between MST and BMI was negative. There was a strong correlation between peripheral muscle strength and age. Peripheral muscle strength also correlated moderately with respiratory muscle strength and lung function.
Conclusion: Children and adolescents with severe asthma had lower maximal and submaximal exercise capacity and reduced peripheral and respiratory muscle strength. These results are slightly below normal and very similar to patients with less severe asthma, allowing us to infer that patients with severe asthma have a good functional condition when the disease is controlled.
Keywords: Asthma; Exercise tolerance; Respiratory muscles; Muscle strength; Pediatrics; Child; Adolescent
| Introduction | ▴Top |
Asthma is characterized by chronic airway inflammation and a history of respiratory symptoms such as wheezing, shortness of breath, chest tightness, cough, and expiratory airflow limitation. A good symptom control and exacerbations can be achieved in most asthma patients with low to moderate doses of inhaled corticosteroids. However, some patients need moderate to high doses of inhaled corticosteroids associated with long-acting beta2-agonist, even after maximum control of comorbidities, environmental exposures, psychosocial factors, adherence to treatment, and correct inhalation technique [1]. These patients have severe asthma, which accounts for approximately 5-10% of all patients with this disease, and 50-60% of the asthma’s medical costs, resulting in a very significant economic impact [2, 3].
Children and adolescents with severe asthma are at increased risk of adverse events, including medication-related side effects and recurrent exacerbations that negatively impact on the quality of life and can be life-threatening [2]. Symptoms and activity limitations [4], as well as greater severity and number of exacerbations [5] are associated with reduction of quality of life. Added to this, the fact that children with asthma have a sedentary lifestyle [6, 7], and limitation for aerobic exercise [8, 9], showing poorer performance than healthy children on maximal [6] or submaximal [7] exercise capacity tests, being the physical limitation directly associated with disease severity [8].
Reduction in peripheral muscle strength has been described in patients with chronic lung disease, predisposing patients to have a more sedentary lifestyle and in a long-term exercise intolerance [8]. However, there is little evidence in muscle strength and peripheral muscle endurance in pediatric severe asthma. In two studies, peripheral muscle strength was normal and was not influenced by disease severity [6, 8]. In another study, the results showed handgrip strength was significantly lower in children with moderate asthma compared with the healthy group and those with mild asthma, demonstrating the influence of asthma severity on muscle strength [10].
Respiratory muscles have been studied more often than peripheral muscles in asthma, but not in severe asthma. In addition, the available evidence on respiratory muscle strength in pediatric asthma is limited and contradictory, given that some studies found no differences between asthmatics and healthy subjects [11-13], and others found a decrease in only one inspiratory or expiratory strength [14, 15].
This study is justified because severe asthma differs from less severe asthma, due to the risk of complications, by the need of higher doses of medications, the increased demand for health services and costs. Therefore, the current study aimed to assess maximal and submaximal exercise capacity, respiratory and peripheral muscle strength and quality of life in children and adolescents with severe asthma. Secondarily, evaluate possible associations between these variables, lung function, demographic and anthropometric data.
| Materials and Methods | ▴Top |
Participants
This was a cross-sectional observational study that evaluated patients with severe asthma, with a consecutively selected sample, in two pediatric referral hospitals in Porto Alegre-RS. We included children and adolescents aged 6 to 18 years diagnosed with severe asthma by an experienced pediatric pulmonologist (steps 4 or 5 of the Global Initiative for Asthma (GINA)) [1]. Patients who had uncontrolled or partially controlled asthma (according to GINA assessment of adults, adolescents and children 6 - 11 years - Box 2-2) [1], who were taking preventive medication irregularly, with musculoskeletal and/or neurological disorders that prevented the comprehension and performance of the tests, diagnosed with pulmonary or systemic arterial hypertension, heart disease and other chronic pulmonary disorders were excluded. Patients who refused (or their guardians refused) to participate also were excluded.
This research was approved by the Research Ethics Committees of the participating institutions: Hospital da Crianca Santo Antonio (2.348.759), Universidade Federal de Ciencias da Saude de Porto Alegre (2.387.650) and Hospital Materno Infantil Presidente Vargas (2.474.352). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. Participants (7 years of age or older) and their legal guardians read, agreed and signed the consent forms.
Logistics
Patients were assessed on the same day of routine appointment with the pulmonologist. The evaluations were performed in the following order: 1) Appointment with a pediatric pulmonologist; 2) Presentation of the research, invitation and signature of consent forms; 3) Completion of the data collection form; 4) 6-min walk test (6MWT); 5) Quality of life questionnaire - asthma module; 6) Manovacuometry; 7) International physical activity questionnaire (IPAQ); 8) Handgrip dynamometry; 9) Modified shuttle test (MST). All evaluations were performed by two trained researchers, and there was a rest time between them.
Maximal exercise capacity
Maximal exercise capacity was performed using the MST [16] and according to European Respiratory Society/American Thoracic Society (ERS/ATS) recommendations [17]. The test has 15 levels in a course of 10 m, delimited by two cones with an inset of 0.5 m from either end. This test requires the patient to walk/run with increasing speeds controlled by a series of pre-recorded signals, ranging from 1.8 to 10.2 km/h and may be interrupted if the patient experiences fatigue or dyspnea and does not reach the distance to the cone two consecutive times [17]. At the beginning of each level, a standardized verbal incentive was offered.
The heart rate, peripheral oxygen saturation and respiratory rate were evaluated at the beginning and end of the test, as the modified Borg scale [18], used to assess the subjective sensation of dyspnea and fatigue of the lower limbs. Total distance walked in meters was measured by counting the total number of shuttles at the end of the test and expressed in meters and compared to the values predicted by the national reference equation [19].
Submaximal exercise capacity
The 6MWT was performed according to the guidelines of the ATS/ERS [17]. The subjects were instructed to walk as far as possible, for 6 min in a 30-m corridor, marked at every 3 m. Interruption or slowing down the rhythm were allowed, if necessary. Standardized phrases of encouragement were reproduced every minute of the course. At the end of the test, the total distance covered in meters was measured and compared to the values predicted by the reference equation [20]. The heart rate, peripheral oxygen saturation and respiratory rate were evaluated at the beginning and end of the test, as the modified Borg scale [18], used to assess the subjective sensation of dyspnea and fatigue of the lower limbs.
Respiratory muscle strength
Evaluation of respiratory muscle strength was performed with a digital manovacuometer (MVD 300®, GlobalMed, Brazil) with variation of ± 300 cm H2O. The maximal expiratory pressure (MEP) was measured from the total lung capacity and the maximal inspiratory pressure (MIP) from the residual volume. The measurements were performed in a seated posture, upright trunk, and with the use of a nose clip.
The test was conducted following the recommendations from the ERS [21]. All guidelines relative to the reproducibility and acceptability of the maneuvers were followed. A minimum of five and a maximum of 10 maneuvers were performed for each type of measured respiratory pressure, containing at least three acceptable (without air leak) and reproducible (that vary by less than 10%) maneuvers. The maneuvers were performed with maximum respiratory efforts, with 1-min intervals and sustained by at least 1 s. For this study, we used the maneuver of the highest value and the results were compared to values predicted by the reference equation [22].
Peripheral muscle strength
To measure the handgrip strength (HS), a hand hydraulic dynamometer (JAMAR®, USA) was used and the measurements were performed following the American Society of Hand Therapists guidelines [23]. The patient was positioned in a chair with feet flat on the floor, shoulder adducted, elbow flexed at 90°, forearm in neutral position and wrist positioned between 0° and 30° extension and between 0° and 15° of ulnar deviation.
The researcher explained the assessment and demonstrated the test’s position, as well as providing verbal encouragement to exercise maximum strength during each measurement. The maneuver was repeated three consecutive times with the dominant hand, and the average of the three measurements was used to compare to the normal values [23].
Lung function
Spirometry was performed by a pediatric pulmonologist using the KoKo spirometer (KoKo® PFT System, United Kingdom) following ATS guidelines acceptability and reproducibility criteria [24]. These data were collected from patients’ medical records when the test had been performed less than 6 months from the day of the evaluation. When there was no spirometry or before 6 months, a new lung function test was scheduled. Lung function was measured without bronchodilator use.
Questionnaires of physical activity and quality of life
The level of physical activity was measured in patients aged 12 years or more using the short version of the IPAQ [25]. The questionnaire contains eight questions related to frequency (days per week) and duration (minutes per day) of physical, vigorous and moderate, activity and walking, categorizing the patient as “very active”, “active”, “irregularly active A”, “irregularly active B” or “sedentary”.
Quality of life was assessed using the Brazilian version of the Pediatric Quality of Life Inventory™ (PedsQL™) 3.0 Asthma Module [26, 27] which contains 28 questions related to asthma in the following domains: asthma symptoms, treatment problems, worry and communication. The questions were answered on a Likert scale from 0 to 4 and, subsequently, the items are linearly inverted and transformed on a scale from 0 to 100, where the highest scores reflected better quality of life. This questionnaire can be answered by the population from 5 to 18 years old and has three versions adapted for each age.
Data analysis
Variables were described by frequency and percentage or mean and standard deviation when normally distributed and median and interquartile range when asymmetric, with normality being verified by the Shapiro-Wilk test. Categorical variables were presented in absolute and relative frequency. For comparisons between observed and predicted values, Student’s t-test was used for parametric domains and Wilcoxon’s W test for nonparametric domains. Pearson and Spearman correlation coefficients were used to measure the correlations between parametric and nonparametric variables, respectively. As an associated analysis, we used the classification of patients into two groups according to the 6MWT and MST performance: those with performance above the 75th percentile (> Q3) versus those with performance below the 75th percentile (< Q3). Comparisons between the two groups were performed using the Mann-Whitney U test.
According to Andrade et al, 2014 [7], the average estimate of the distance covered in the 6MWT of children and adolescents with severe asthma was 433.7 ± 124.2 m. With a maximum error of 45 m from this estimate, a significance level of 5% and a test power of 80%, the sample was estimated at 29 participants. Data were analyzed using the SPSS software (IBM SPSS Statistics for Windows, version 25., NY, USA) and the significance level was 5% (P < 0.05).
| Results | ▴Top |
We identified 391 patients aged 6 to 18 years old with a diagnosis of asthma. Of these, 78 (20.0%) were classified in step 4 and none reached step 5. Of the 78 patients with severe asthma, 20 were excluded because they had some associated disease, 17 had an uncontrolled disease or were misusing preventive medication, seven did not attend on the day of evaluation and four refused (or their guardians refused) to participate. Thus, 30 patients were recruited, and their demographic, anthropometric and pulmonary function data are described in Table 1.
 Click to view | Table 1. Clinical Characteristics of Patients With Severe Asthma |
Of these 30 patients, 28 (93.3%) were of adequate height and two (6.7%) had short stature. In the terms of body mass index (BMI), 16 (53.3%) patients were eutrophic, five (16.7%) were overweight, eight (26.7%) were obese, and one (3.3%) had severe obesity [28]. The lung function was performed in 23 patients because the others could not perform an adequate curve or missed the exam day. Regarding comorbidities, 26 (86.7%) of the subjects had a clinical diagnosis of rhinitis (all with controlled symptoms), 11 (36.7%) of atopic dermatitis, four (14.3%) of food allergy and two (6.7%) of gastroesophageal reflux. Passive smoking was observed in 11 (36.7%) patients, and none of the patients were active smokers. Eleven patients were 12 years old or older and answered the IPAQ, most of them (54.3%) being active.
Table 2 shows the performance in the maximal and submaximal exercise capacity, and respiratory and peripheral muscle strength tests, compared to the values predicted by reference equations or normal values. Patients underperformed but reached more than 80% of what was expected in all evaluations. No adverse events occurred during or at the end of either exercise test, except for two patients who presented wheezing after MST, requiring the use of bronchodilators.
 Click to view | Table 2. Comparison Between Predicted and Measured Values of the Tests |
Figure 1 shows the correlations between MST, age, BMI, lung function, respiratory and peripheral muscle strength. There was a moderate correlation between MST and both age and BMI, the latter being negative, meaning that the higher the BMI, the worse the performance on the maximal exercise test.
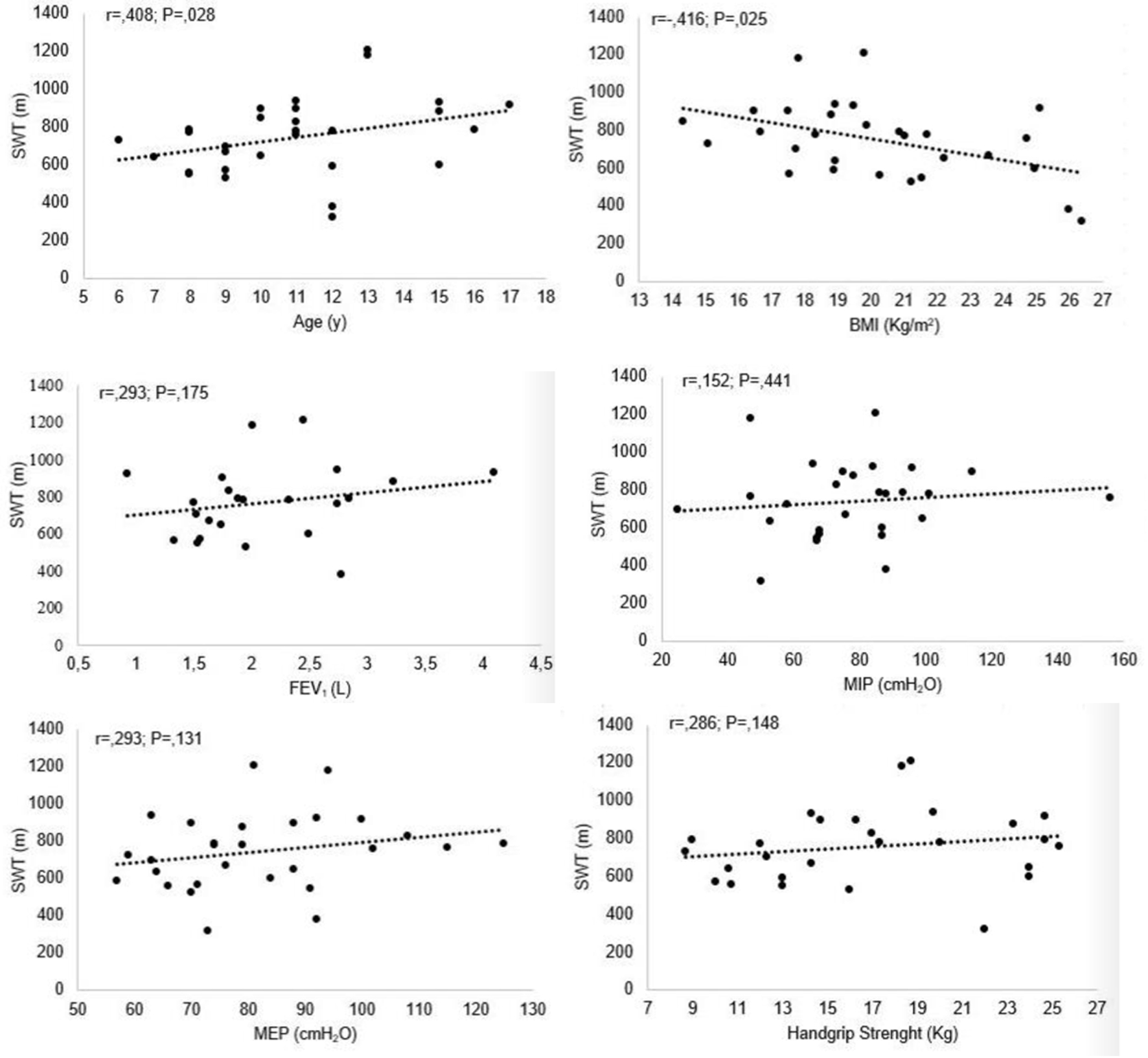 Click for large image | Figure 1. Correlation between MST and age, BMI, pulmonary function test, respiratory and peripheral muscle strength. MEP: maximal expiratory pressure; MIP: maximal inspiratory pressure; MST: modified shuttle test; BMI: body mass index. |
A moderate correlation was found between peripheral muscle strength and MIP (r = 0.487; P = 0.010) and MEP (r = 0.498; P = 0.008). Moreover, there was a strong correlation between peripheral muscle strength and age (r = 0.777; P < 0.001) and moderate with BMI (r = 0.513; P = 0.005), forced vital capacity (FVC) (r = 0.506; P = 0.016) and forced expiratory volume at first second of FVC (FEV1) (r = 0.484; P = 0.022). Finally, MEP correlated moderately with age (r = 0.425; P = 0.024), BMI (r = 0.432; P = 0.022) and MIP (r = 0.393; P = 0.038).
In the subgroup analysis, patients were classified into two distinct groups according to performance on the 6MWT and MST: those with performance above the 75th percentile (> Q3) versus those with performance up to the 75th percentile (< Q3). In the 6MWT, patients > Q3 achieved a result ≥ 94% of the predicted distance traveled. This group showed significantly higher values of FVC (118% versus 103.9%; P = 0.018) and FEV1 (120% versus 96%; P = 0.008) compared to < Q3 (Fig. 2). The same analysis was performed for MST: group > Q3 had a performance ≥ 93% of the predicted, but there was no difference about the other percentiles in any evaluated variable.
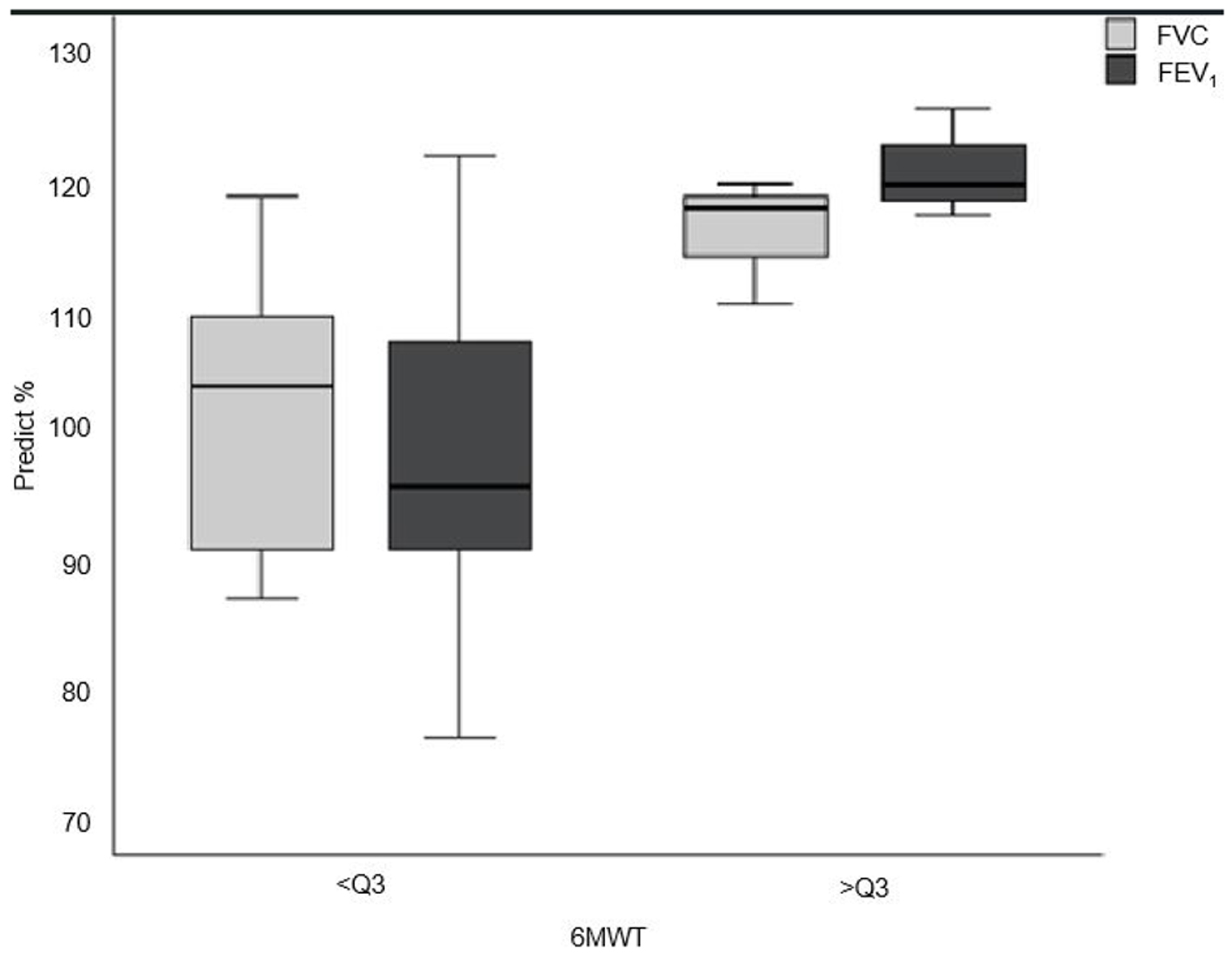 Click for large image | Figure 2. Group with < Q3 percentile compared to > Q3 on the 6MWT in relation to FVC and FEV1. 6MWT: 6-min walk test; Q3: 75th percentile. FEV1: forced expiratory volume at first second of FVC; FVC: forced vital capacity. |
Regarding quality of life, all significant correlations were negative, indicating that older patients with higher BMI, MEP, and greater peripheral muscle strength had less quality of life. The correlations are described in Table 3.
 Click to view | Table 3. Correlations Between PedsQL Asthma and Age, BMI, Maximal and Submaximal Exercise Capacity, Peripheral and Respiratory Muscle Strength |
| Discussion | ▴Top |
Children and adolescents with severe asthma showed a lower maximal and submaximal exercise capacity and reduced peripheral and respiratory muscle strength. Pulmonary function was normal and quality of life was worse in older patients.
The results of the present study demonstrate a reduction in the maximal exercise capacity evaluated by the MST. Similar to our results, Reimberg et al, 2018 [6] also found that children and adolescents who had asthma with different severities covered 82% of the predicted distance. Thus, our results suggest that patients with severe asthma had reduced maximal exercise capacity, similar to patients with general asthma.
The MST has been used as an alternative test to evaluate the maximal exercise capacity because it is an easily accessible test, safe for the clinical environment, and has an important correlation with peak oxygen uptake (VO2peak) in children and adolescents with asthma [29, 30]. In the study by Lanza et al, 2018 [29], the MST achieved higher VO2peak and heart rate values than cardiopulmonary exercise testing (CPET), with a strong correlation between the VO2peak predicted by the MST equation and the VO2peak measured by CPET. Similarly, a strong correlation was found between the MST and VO2peak measured through CPET in children and adolescents with severe therapy-resistant asthma in the study by Schiwe et al, 2019 [30].
In the 6MWT, children and adolescents in this study performed better than patients with severe asthma evaluated by Andrade et al, 2014 [7]. However, the result was lower when compared to patients with mild [14] or all severities of asthma [31].
In subgroup analysis, patients with better results in the 6MWT (above the 75th percentile), had significantly better FVC and FEV1 values. Despite being an important finding, its clinical relevance needs to be questioned because the patients in this study had normal lung function. However, in the future, a group of patients with impaired lung function may be studied to verify the hypothesis that FVC and FEV1 could account for worse performance in the 6MWT and even in the maximal test.
Studies [32-34] have shown that children with asthma participate less frequently in physical activity because of the fear of both parents and children that exercise may trigger an asthma attack. Dyspnea during exercise may be mistakenly attributed to asthma when it is a normal consequence of exertion or the result of a lack of physical fitness. Thus, children with asthma do not participate in physical exercise, leading to a reduction in cardiorespiratory conditioning [35]. In our study, more than half of the patients aged 12 years and older were classified as active in IPAQ, and the remaining as insufficiently active. This result is lower than the study by Basso et al, 2013 [36], with adolescents, where 63.1% were active or very active. However, in the cited study, all patients evaluated had a diagnosis of mild asthma, in contrast to the patients in this study with severe asthma.
Regarding respiratory muscle strength, most previous studies [11-13] showed no differences between healthy and asthmatic children and, when absolute values were observed, better results were achieved than our patients. However, none of the studies have been carried out in patients with severe asthma, showing that asthma severity may influence respiratory muscle strength. A possible explanation for the lower respiratory strength of our patients with severe asthma is the changes that these muscles undergo due to the need of having to do a greater work to overcome the increased expiratory resistance caused by airway obstruction. At the same time, it is disadvantaged by pulmonary hyperinflation caused by air trapping, which leads to shortening of the inspiratory muscles and puts them at a mechanical disadvantage [37, 38]. These changes damage the kinetics of these muscles and may reduce their contraction strength.
Measurement of muscle strength through handgrip has been used as a general indicator for overall muscle strength [39] and as a complementary instrument in asthma monitoring in clinical practice [10]. In the study by Latorre-Roman et al, 2014 [10], it was observed that children with asthma had a reduction of approximately 22% in handgrip strength compared to healthy ones and that the strength was about 13.6% lower in patients with moderate asthma compared to mild asthma. In the present study, we found higher values than the previously mentioned study, even though they are patients with severe asthma. Similarly to the study by Latorre-Roman et al, 2014 [10], a positive correlation between handgrip strength and both BMI and FEV1 was observed.
Approximately half of the patients in this study are overweight or obese, very similar to those found by Glazebrook et al, 2006 [33]. Obesity is one of the factors directly related to the increased risk and severity of asthma, and asthma is more common in obese than in thin people [3]. We also observed a negative correlation between BMI and distance achieved in the MST, meaning that the higher the BMI, the worse the performance on the maximal exercise test. However, we did not find the same correlation between BMI and 6MWT in this study, as found by Rastogi et al, 2012 [40], where obesity contributed to worse performance of asthmatic adolescents in submaximal exercise capacity.
Quality of life is related to the level of control and severity of asthma in children and adolescents [34, 41]. In the present study, patients scored around 70 in the domains related to symptoms and worry with the disease, while in the domains related to problems with treatment or communication, the score was higher than 80. These findings are similar to studies [26, 42] with children and adolescents with different severities of asthma, that is, the patients with severe asthma in this study have a similar quality of life to patients with less severe asthma. We hypothesized that the reason for this finding is due to the fact that our patients maintained good clinical follow-up and disease control, which meets a better quality of life outcomes. All significant correlations found were negative, which means that the older and higher peripheral muscle strength, the worse the quality of life reported, which probably occurred due to the greater difficulty of understanding and reflection of younger children regarding the questions.
Our study has some limitations, such as the loss of some patients in the lung function test and the impossibility to assess the level of physical activity in the patients below 12 years old. Another limitation is that for comparison with normality, reference values published in the literature were used and not a group of healthy children and adolescents; however, these equations have been widely accepted in the literature.
In conclusion, the results of our study demonstrate that children and adolescents with severe asthma had lower maximal and submaximal exercise capacity and lower respiratory and peripheral muscle strength. Quality of life was lower in older patients, higher BMI and greater muscle strength. These results are slightly below normal and very similar to patients with less severe asthma, allowing us to infer that patients with severe asthma have a good functional condition when the disease is controlled.
Acknowledgments
Thanks to pediatric pulmonologist Andre Longhi for the opportunity provided and for helping with patients.
Financial Disclosure
This research did not receive any funding source.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Participants (7 years of age or older) and their legal guardians read, agreed and signed the consent forms.
Author Contributions
NM: conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article and final approval the version to be submitted. BB: acquisition of data, revising the article and final approval the version to be submitted. HTM and GBF: conception and design of the study, analysis and interpretation of data, revising the article and final approval the version to be submitted. JLL: conception and design of the study, analysis and interpretation of data, drafting the article and final approval the version to be submitted.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2022. Available from: www.ginasthma.org.
- Fitzpatrick AM. Severe asthma in children: lessons learned and future directions. J Allergy Clin Immunol Pract. 2016;4(1):11-19.
doi pubmed - Tashiro H, Shore SA. Obesity and severe asthma. Allergol Int. 2019;68(2):135-142.
doi pubmed - Hossny E, Caraballo L, Casale T, El-Gamal Y, Rosenwasser L. Severe asthma and quality of life. World Allergy Organ J. 2017;10(1):28.
doi pubmed - Luskin AT, Chipps BE, Rasouliyan L, Miller DP, Haselkorn T, Dorenbaum A. Impact of asthma exacerbations and asthma triggers on asthma-related quality of life in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2014;2(5):544-552.e541-542.
doi pubmed - Reimberg MM, Pachi JRS, Scalco RS, Serra AJ, Fernandes L, Politti F, Wandalsen GF, et al. Patients with asthma have reduced functional capacity and sedentary behavior. J Pediatr (Rio J). 2020;96(1):53-59.
doi pubmed - Andrade LB, Silva DA, Salgado TL, Figueroa JN, Lucena-Silva N, Britto MC. Comparison of six-minute walk test in children with moderate/severe asthma with reference values for healthy children. J Pediatr (Rio J). 2014;90(3):250-257.
doi pubmed - Villa F, Castro AP, Pastorino AC, Santarem JM, Martins MA, Jacob CM, Carvalho CR. Aerobic capacity and skeletal muscle function in children with asthma. Arch Dis Child. 2011;96(6):554-559.
doi pubmed - Lochte L, Angermann M, Larsson B. Cardiorespiratory fitness of asthmatic children and validation of predicted aerobic capacity. Clin Respir J. 2009;3(1):42-50.
doi pubmed - Latorre-Roman PA, Navarro-Martinez AV, Manas-Bastidas A, Garcia-Pinillos F. Handgrip strength test as a complementary tool in monitoring asthma in daily clinical practice in children. Iran J Allergy Asthma Immunol. 2014;13(6):396-403.
- Marcelino AM, da Cunha DA, da Cunha RA, da Silva HJ. Respiratory muscle strength in asthmatic children. Int Arch Otorhinolaryngol. 2012;16(4):492-496.
doi pubmed - Oliveira CM, Lanza Fde C, Sole D. Respiratory muscle strength in children and adolescents with asthma: similar to that of healthy subjects? J Bras Pneumol. 2012;38(3):308-314.
doi pubmed - Heinzmann-Filho JP, Vendrusculo FM, Woszezenki CT, Piva TC, Santos AN, Barcellos AB, Vagliatti BB, et al. Inspiratory muscle function in asthmatic and healthy subjects: influence of age, nutrition and physical activity. J Asthma. 2016;53(9):893-899.
doi pubmed - Soares AAA, Barros CM, Santos CGC, Dos Santos MRA, Silva JRS, Silva Junior WMD, Simoes SM. Respiratory muscle strength and pulmonary function in children with rhinitis and asthma after a six-minute walk test. J Asthma. 2018;55(3):259-265.
doi pubmed - de Andrade TCQ, Feitosa LAdS, Carvalho LdA, Marinho PEdM, de Andrade AdFD. Influencia dos corticosteroides inalatorios nas pressoes respiratorias maximas de criancas escolares asmaticas. Fisioter Mov. 2012;25(1):67-72.
doi - Bradley J, Howard J, Wallace E, Elborn S. Validity of a modified shuttle test in adult cystic fibrosis. Thorax. 1999;54(5):437-439.
doi pubmed - Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, McCormack MC, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
doi pubmed - Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
doi pubmed - Lanza Fde C, Zagatto Edo P, Silva JC, Selman JP, Imperatori TB, Zanatta DJ, de Carvalho LN, et al. Reference equation for the incremental shuttle walk test in children and adolescents. J Pediatr. 2015;167(5):1057-1061.
doi pubmed - Geiger R, Strasak A, Treml B, Gasser K, Kleinsasser A, Fischer V, Geiger H, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150(4):395-399.e391-392.
doi pubmed - Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, Dube BP, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53(6):1801214.
doi pubmed - Lanza FC, de Moraes Santos ML, Selman JP, Silva JC, Marcolin N, Santos J, Oliveira CM, et al. Reference equation for respiratory pressures in pediatric population: a multicenter study. PLoS One. 2015;10(8):e0135662.
doi pubmed - McQuiddy VA, Scheerer CR, Lavalley R, McGrath T, Lin L. Normative values for grip and pinch strength for 6- to 19-year-olds. Arch Phys Med Rehabil. 2015;96(9):1627-1633.
doi pubmed - Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338.
doi pubmed - Matsudo S, Araujo T, Matsudo V, Andrade D, Andrade E, Oliveira LC, et al. Questionario Internacional de Atividade Fisica (IPAQ): Estudo de validade e reprodutibilidade no Brasil. Atividade Fisica & Saude. 2001;6(2):5-18.
- Varni JW, Burwinkle TM, Rapoff MA, Kamps JL, Olson N. The PedsQL in pediatric asthma: reliability and validity of the Pediatric Quality of Life Inventory generic core scales and asthma module. J Behav Med. 2004;27(3):297-318.
doi pubmed - Monteiro FP, Sole D, Wandalsen G. Quality of life of asthmatic children and adolescents: Portuguese translation, adaptation, and validation of the questionnaire "Pediatric Quality of Life (PedsQL) Asthma Module". J Asthma. 2017;54(9):983-989.
doi pubmed - W. H. O. Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76-85.
doi pubmed - Lanza FC, Reimberg MM, Ritti-Dias R, Scalco RS, Wandalsen GF, Sole D, van Brussel M, et al. Validation of the modified shuttle test to predict peak oxygen uptake in youth asthma patients under regular treatment. Front Physiol. 2018;9:919.
doi pubmed - Schiwe D, Heinzmann-Filho JP, Schindel CS, Gheller MF, Campos NE, Pitrez PM, Donadio MVF. Modified shuttle test distance correlates with peak oxygen uptake in children and adolescents with severe therapy-resistant asthma. Front Physiol. 2019;10:1245.
doi pubmed - Latorre-Roman PA, Navarro-Martinez AV, Garcia-Pinillos F. The effectiveness of an indoor intermittent training program for improving lung function, physical capacity, body composition and quality of life in children with asthma. J Asthma. 2014;51(5):544-551.
doi pubmed - Williams B, Hoskins G, Pow J, Neville R, Mukhopadhyay S, Coyle J. Low exercise among children with asthma: a culture of over protection? A qualitative study of experiences and beliefs. Br J Gen Pract. 2010;60(577):e319-326.
doi pubmed - Glazebrook C, McPherson AC, Macdonald IA, Swift JA, Ramsay C, Newbould R, Smyth A. Asthma as a barrier to children's physical activity: implications for body mass index and mental health. Pediatrics. 2006;118(6):2443-2449.
doi pubmed - Furtado PR, Maciel ACC, Barbosa RRT, Silva A, Freitas DA, Mendonca K. Association between quality of life, severity of asthma, sleep disorders and exercise capacity in children with asthma: a cross-sectional study. Braz J Phys Ther. 2019;23(1):12-18.
doi pubmed - Craig TJ, Dispenza MC. Benefits of exercise in asthma. Ann Allergy Asthma Immunol. 2013;110(3):133-140.e132.
doi pubmed - Basso RP, Jamami M, Labadessa IG, Regueiro EM, Pessoa BV, Oliveira AD, Jr., Di Lorenzo VA, et al. Relationship between exercise capacity and quality of life in adolescents with asthma. J Bras Pneumol. 2013;39(2):121-127.
doi pubmed - Weiner P, Azgad Y, Ganam R, Weiner M. Inspiratory muscle training in patients with bronchial asthma. Chest. 1992;102(5):1357-1361.
doi pubmed - Weiner P, Suo J, Fernandez E, Cherniack RM. Hyperinflation is associated with reduced strength and efficiency of the respiratory muscles in asthmatic and normal subjects. Chest. 1990;97(3 Suppl):69S-70S.
doi - Wind AE, Takken T, Helders PJ, Engelbert RH. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur J Pediatr. 2010;169(3):281-287.
doi pubmed - Rastogi D, Khan UI, Isasi CR, Coupey SM. Associations of obesity and asthma with functional exercise capacity in urban minority adolescents. Pediatr Pulmonol. 2012;47(11):1061-1069.
doi pubmed - Matsunaga NY, Ribeiro MA, Saad IA, Morcillo AM, Ribeiro JD, Toro AA. Evaluation of quality of life according to asthma control and asthma severity in children and adolescents. J Bras Pneumol. 2015;41(6):502-508.
doi pubmed - Khoshkhui M, Jafari P, Afrasiabi M, Orooj M, Kashef S. Level of agreement between children with asthma and their parents on quality of life. Iran J Med Sci. 2016;41(2):86-93.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
