| International Journal of Clinical Pediatrics, ISSN 1927-1255 print, 1927-1263 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Int J Clin Pediatr and Elmer Press Inc |
| Journal website https://www.theijcp.org |
Original Article
Volume 11, Number 1, March 2022, pages 2-8
Magnetic Resonance Imaging of the Caudal Epidural Space: Implications for the Clinical Practice Regional Anesthesia in Infants and Children
Tariq M. Wania, c, Odai Khasmaha, Carmen Simiona, Saif Rehmana, Joseph D. Tobiasb
aDepartment of Anesthesia, Sidra Medicine, Doha, Qatar
bDepartment of Anesthesiology & Pain Medicine, Nationwide Children’s Hospital and The Ohio State University, Columbus, Ohio, USA
cCorresponding Author: Tariq M. Wani, Department of Anesthesiology, Sidra Medicine, Doha, Qatar
Manuscript submitted February 10, 2022, accepted March 22, 2022, published online March 29, 2022
Short title: Caudal Epidural Anesthesia
doi: https://doi.org/10.14740/ijcp480
| Abstract | ▴Top |
Background: Neuraxial blockade involving the lumbar and caudal space spine is commonly employed in pediatric anesthesia. Although the incidence of complications is low, the consequences including total spinal block, spinal cord or nerve injury, and post-dural puncture headache may be significant. Information regarding the vertebral level at which the spinal cord terminates and the distance from the skin to the end of the dural sac (DS) may guide clinical practices. The present study measures various distances of the lumbar and caudal epidural space in children using magnetic resonance imaging (MRI).
Methods: This retrospective study analyzed sagittal MR images of the lumbar spine of children ranging in age from birth to 8 years. Measurements included the level of termination of the spinal cord and DS; the distance from the end of the spinal cord to the end of the DS, the sacrococcygeal membrane (SCM), and the skin; and the distance from the end of the DS to the SCM and the skin. Descriptive statistics included mean, median, range, standard deviation, interquartile range, and confidence intervals.
Results: The study cohort included 91 patients, ranging in age from birth to 8 years. There was no significant difference in the variables between males and females. Using patient age, height, weight and body surface area (BSA) as variables, there was a statistically significant relationship of age with all measured parameters. There was significant interpatient variability despite grouping the data in small groups with regards to age, height, and weight. The variance inflation factor (VIF) between age and BSA was 3.51, not indicative of any collinearity issues.
Conclusion: The wide variability in measurements among studied groups, despite dividing them into smaller groups with regards to age, weight or height, would make it difficult to develop unifying formulas based on physical and age characteristics. The spinal cord ended at L1 in children more than 12 months of age, which contradicts earlier studies suggesting its lower position at L2-3. The terminal end of the spinal cord was found at L2 in the majority of the patients less than 12 months of age.
Keywords: Dural sac; Spinal cord; Sacrococcygeal membrane; Caudal epidural anesthesia
| Introduction | ▴Top |
Understanding caudal and lumbar vertebral anatomy is crucial for successful and safe neuraxial blockade in children [1, 2]. Caudal and lumbar neuraxial blockades (spinal and epidural anesthesia) are two of the most commonly performed regional anesthetic techniques in infants and children. Two European multi-center studies showed that these blocks accounted for 27-49% of all regional anesthesia techniques [3, 4]. Similar data are available from a North American database with these blocks accounting for 44% of perioperative regional anesthesia techniques [5, 6]. Although the incidence of complications with neuraxial blockade is low, the consequences may be significant including total spinal anesthesia, spinal cord or nerve injury, and post-dural puncture headache [7-10]. The incidence of unintended dural puncture at the lumbar level is approximately 86 in 10,000 and 10 in 10,000 at the caudal level [6].
Information regarding the anatomy of the caudal and lumbar vertebral space may be useful in guiding safe practices during regional anesthesia in infants and children. Prior studies have investigated the relevant anatomy of the thoracic, lumbar, and sacral regions using imaging techniques (magnetic resonance imaging (MRI) or ultrasound) and cadaveric specimens with the development of formulas for calculating various distances such as the sacrococcygeal membrane (SCM) to the dural sac (DS) [11-13]. The present study re-evaluates the anatomical depths and related equations by measuring the distance from the skin and the SCM to the DS as well as the position of the end of the spinal cord and DS in relationship to the vertebral levels using MR images in the pediatric population.
| Materials and Methods | ▴Top |
This study was approved by the Institutional Review Board of Sidra Medicine (Doha, Qatar). It was conducted in accordance with the guidelines outlined in the Declaration of Helsinki. This retrospective observational study included MR images from January 2018 to January 2020. From the various images available, a single sagittal image was chosen for all measurements. The study analyzed sagittal T2-weighted MR images of the lumbar and caudal spine of pediatric patients in the supine position from birth to 8 years of age. Patients with a history of vertebral anomalies including scoliosis, tethered cord, spina bifida, myelomeningocele, tumors of the spinal cord or vertebral bodies, neurologic deficits, and metastatic spinal disease or poor image quality were excluded from the study. MRI was generally obtained as part of the initial workup for patients with lymphoma or leukemia and following placement of a spica cast for a congenital dislocated hip.
An internal measurement device was used for the measurements. The measurements included: 1) the vertebral level at which the spinal cord and the DS ended; 2) the distance from the end of the spinal cord to the end of the DS, the SCM, and the skin; and 3) the distance from the end of the DS to the SCM and the skin (Figs. 1-3). For spinal cord and DS localization, the specific vertebral level was chosen if the spinal cord or DS terminated at any point along those vertebrae down to the lower margin of the intervertebral ligament. The same technique was used for all other spinal cord measurements, knowing that the interspinous ligament corresponds to intervertebral ligaments at the lumbar levels. The skin-to-DS depth was measured using the shortest distance in attempt to most closely replicate the trajectory of a needle used for caudal epidural blockade. The measurements were taken by two of the authors independent of each other and were verified by a co-author. The authors reviewed any discrepancy between the measurements. The investigators were blinded to the age of the patients.
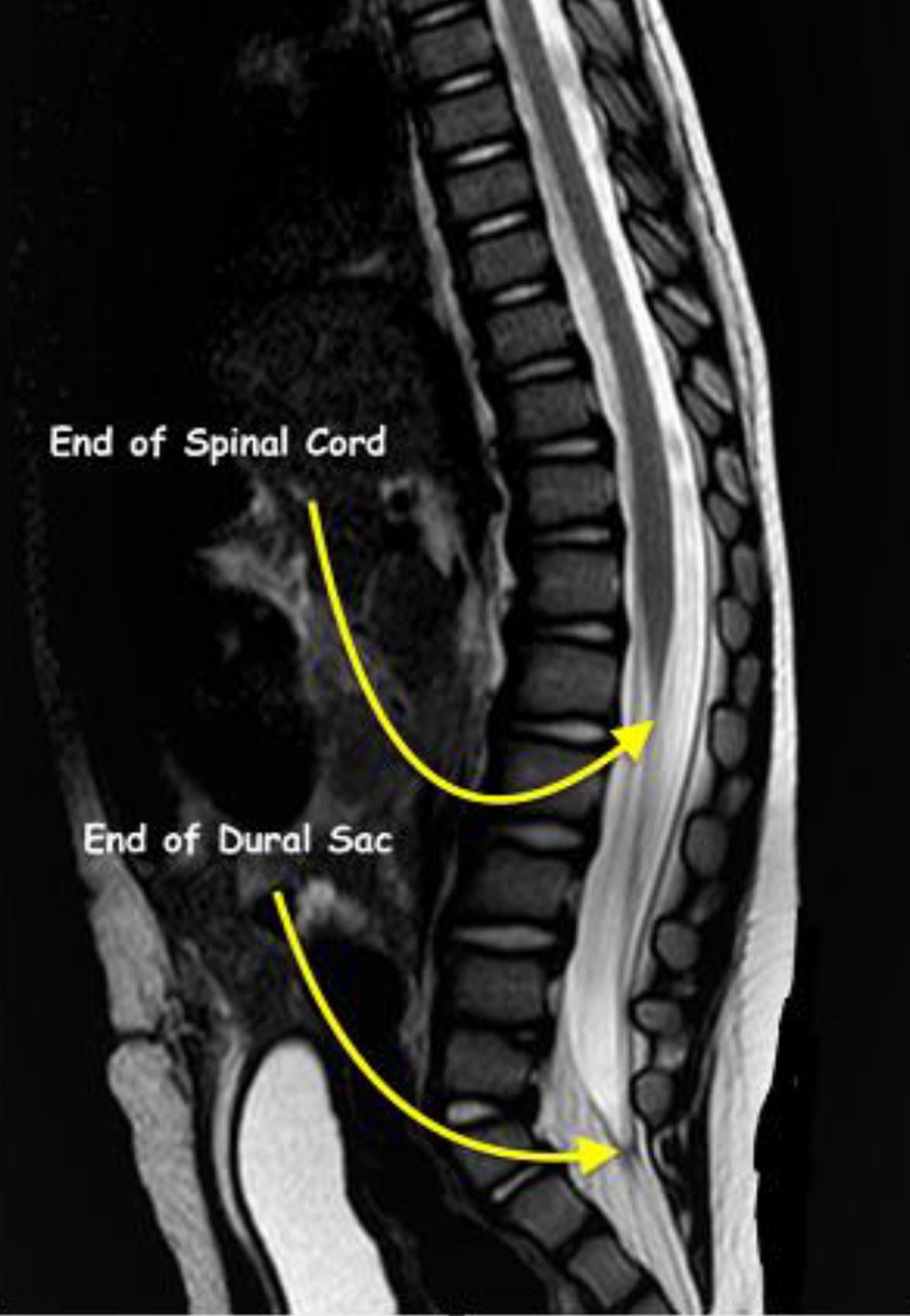 Click for large image | Figure 1. Magnetic resonance imaging showing termination of the spinal cord at L1 and the dural sac at S2 in a 1-year-old patient from the study cohort. |
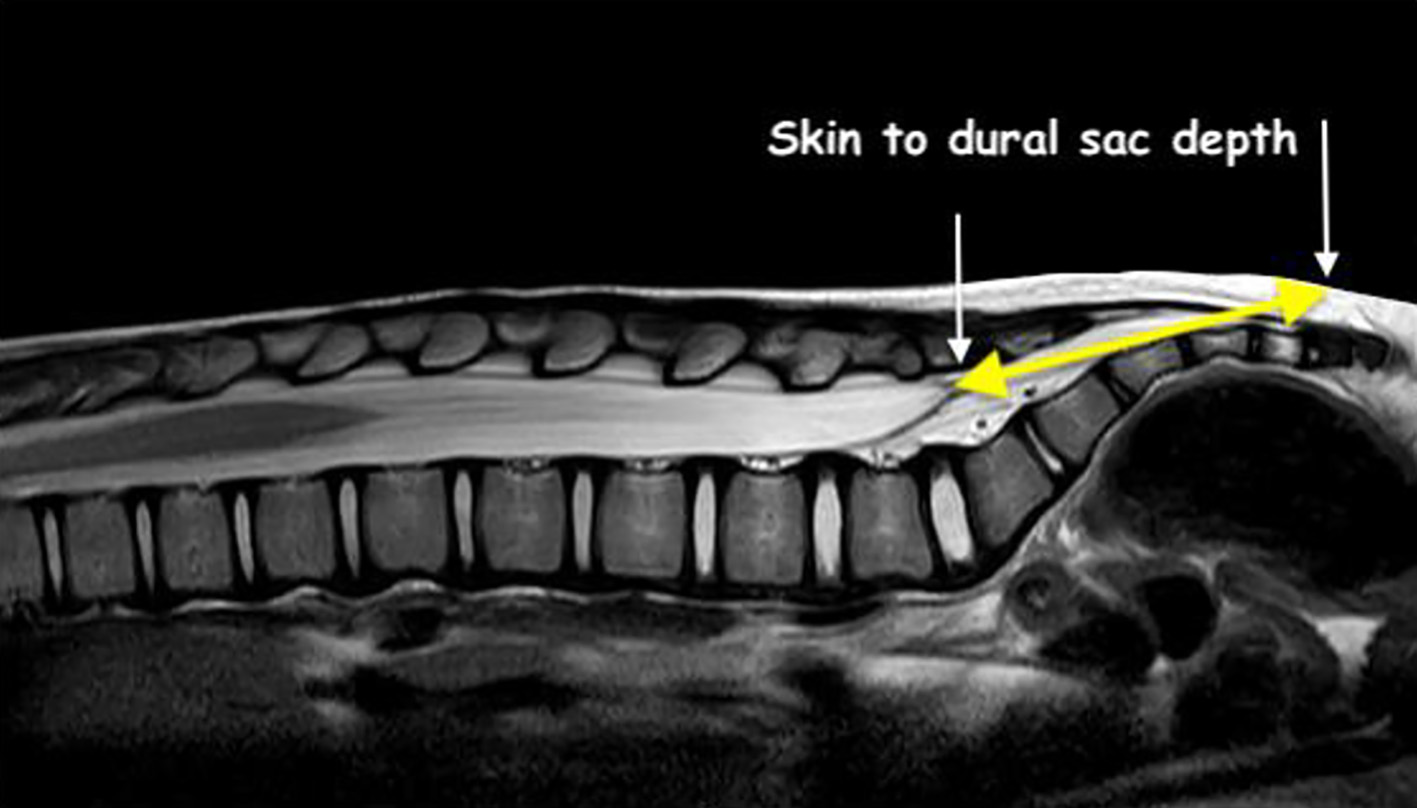 Click for large image | Figure 2. Technique for measurement of the distance from skin to the termination of the dural sac (yellow line). |
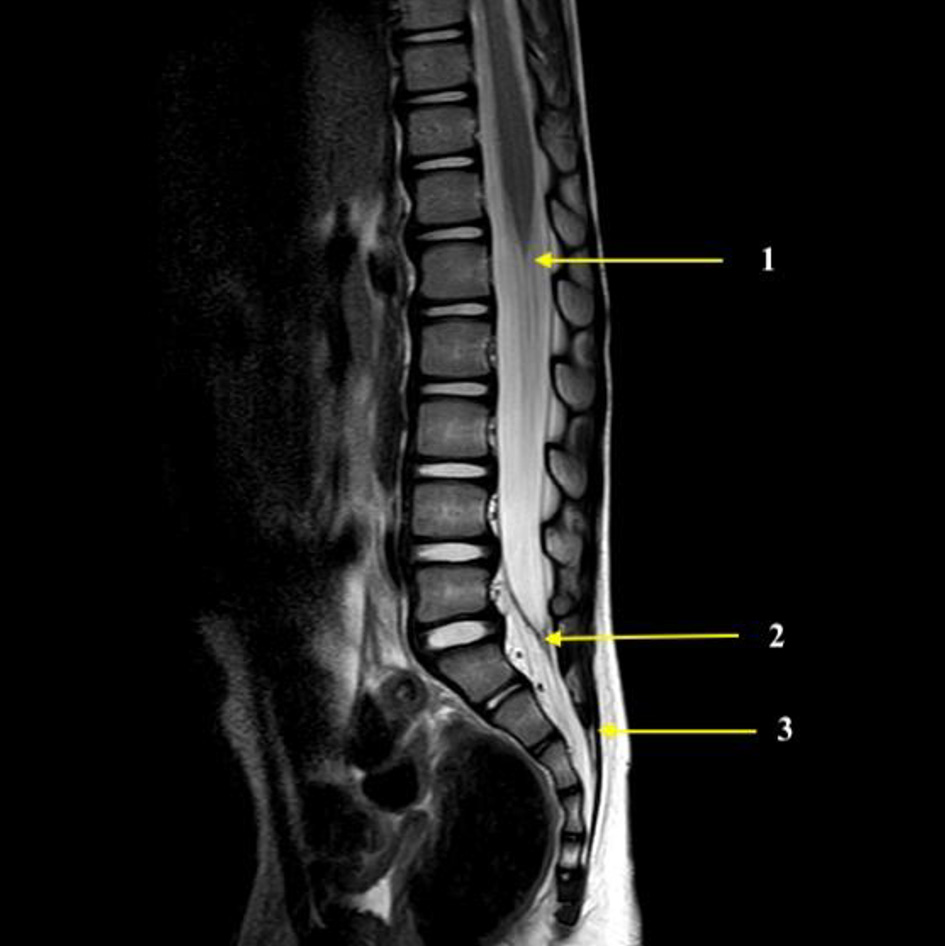 Click for large image | Figure 3. Magnetic resonance imaging showing the end of the spinal cord (1), the termination of the dural sac (2), and the sacrococcygeal ligament (3). These structures were used for the measurements made in the current study. |
Statistical analysis
Descriptive statistics including mean and standard deviation (SD) were computed for all demographic and physical measurement variables. The values were also stratified and compared between males and females using an independent t-test. Measurements stratified by age, mean, median, range, SD, interquartile range (IQR), and confidence intervals (CIs) were calculated. Univariate and multivariate linear regression models were applied with the measurements being the dependent variables and age and body surface area (BSA) as the independent variables. Unadjusted and adjusted slopes, standard errors (SEs), P-values and the coefficients of determinations (R2) were reported. BSA was computed for the participants using Mosteller formula. Variance inflation factors (VIFs) were computed to assess the possible collinearity between age and BSA in those models. Derived measurements to calculate the distance from the SCM to the DS using Lee formula (distance in mm = 25 × BSA) and Adewale formula (distance in mm = 13 + 15 × BSA) were compared with the actual measurements of our study using the paired t-test. Relative % differences were also computed. A P value of 0.05 or less was considered statistically significant. All the analyses were performed using SAS 9.4 (Cary, NC, USA).
| Results | ▴Top |
The study cohort included 91 pediatric patients, 47 males and 44 females. Demographic and physical data including age, height, weight, and BSA are summarized in Table 1. There were no significant differences in the demographics and physical variables (age, height, weight, and BSA) between males and females. There were no significant differences in the five measurements (distances measured) between males and females (Table 1). The measurements stratified by age are summarized in Table 2. The mean of all the measurements studied (DS to SCM, DS to skin) showed a progressive increase with age. There was significant interpatient variability resulting in wide ranges, IQRs, and CIs in each age-specific group.
 Click to view | Table 1. Patient Demographic and Physical Data |
 Click to view | Table 2. Descriptive Statistics With Regards to Age |
Linear regression models presented in Table 3 showed that even after adjusting for the BSA of the participants, age was positively associated with all five measurements. For example, even after adjusting for the BSA of the participants, for every 1 month increase in age, the measurement between the spinal cord and DS increased by 0.57 mm, that between the spinal cord to skin increased by 0.68 mm and that between the DS to skin increased by 0.12 mm. BSA was also independently and significantly positive with all measurements except for the one from the DS to sacral hiatus (P = 0.170). The coefficient of determination for the five multivariate models ranged between 0.48 and 0.84. The VIF between age and BSA was 3.51, not indicative of any collinearly issues in these models (Table 3).
 Click to view | Table 3. Univariate and Multivariate Linear Regression to Assess the Association Between Age and BSA on Measured Distances |
There was a significant difference in the DS to sacral hiatus distance measured in this study and the one obtained using Lee’s formula (23.9 vs. 16.4 mm, P < 0.001). On the other hand, the difference with the distance obtained using Adewale formula did not reach statistical significance (23.9 vs. 22.9 mm, P = 0.061). Mean percent changes between the measured distance and the ones obtained by Lee ranged between -21.2% and 358.0% with a mean of 154.3±62.9%, whereas the ones obtained using Adewale formula ranged between -67.1% and 54.8% with an average of -3.23±22.9%.
In the cohort of 91 patients studied, the spinal cord ended most commonly at the L1 vertebral level. The spinal cord ended at L1 in 63 patients (69.2%), L2 in 23 patients (25.2%), L3 in two patients (2.3%) and T12 in three patients (3.3%). In patients less than 12 months, the spinal cord ended at or below the L2 vertebral level more commonly than in the overall cohort (73.3% versus 27.5%). The DS tapered at the level of S2 vertebrae in the majority of the patients (68 of 91 patients or 74.7%). In 22 (24.1%) patients, the DS tapered at the S1 vertebral level and at the L5 level in one patient. In patients less than 24 months of age, the DS terminated at S2 in a higher percentage of the patients (28 of 32 or 87.5%) compared to children who were more than 24 months of age (39 of 59 patients, 66.1%).
| Discussion | ▴Top |
Using MRI, the current study attempts to define anatomical dimensions and depths of the caudal and lumbar area to facilitate the safe and effective performance of regional anesthetic techniques of the caudal and lumbar areas in infants and children. The measurements were made along planes intended to simulate needle trajectory. Although five different measurements were determined, we would postulate that for a clinician performing a caudal epidural block, the most important measurement would be the distance from the skin to the end of the DS as this may prevent inadvertent dural puncture. Even though we observed a progressive increase in all measurements with an increase in age, the data revealed significant interpatient variability in regard to the distances when grouping patients according to age. Even when dividing the study population into smaller groups (10 groups based on gradations of age), similar findings were noted with significant ranges and wide CIs. These results suggest that development of a specific formula to guide maximum depth of needle insertion is likely not feasible and that individualized care is necessary with attention to limit needle insertion to the minimum distance necessary after puncture of the SCM. Despite these findings, it is reassuring to note that dural puncture is extremely uncommon during placement of a caudal epidural blockade.
As the distances included in the current study are small, interpatient variability and any error in the calculation of depth could increase the risk of accidental dural puncture during the placement of a caudal epidural block. Earlier studies based on MRI measurements from the upper edge of the SCM to the DS have proposed formulas, based on regression analysis of the data, for the calculation of the distance required for needle insertion [11, 13]. Lee et al devised a simple formula to estimate the distance in millimeters from the SCM to the DS: 25 × BSA, whereas Adewale et al proposed that the best predictor of distance between the upper margin of the SCM and DS in millimeters was 13 + (15 × BSA) based on multiple linear regression (stepwise technique) [11, 13].
The interpatient variability noted in our measurements would question the generalized applicability of these formulas. This is especially true since our regression analysis showed an independent effect of age on such measurement, something that neither Lee nor Adewale use in their computation. Part of the discrepancy from the study of Lee et al may be explained by the fact that the patients in his study had a skin dimple, although none of the patients was found to have any abnormality of the spine or the spinal cord [13]. Fourteen of the 41 patients studied by Adewale et al had spinal or skeletal pathologies [11]. In both studies, the depth from the skin to the SCM was not measured nor was the skin to DS distance, the latter of which we believe may be the most clinically relevant distance.
The secondary aim of our study was to determine the vertebral level at which the spinal cord and the DS ended in the different age groups. When considering the entire cohort, the spinal cord ended at L1 in most of the patients, although this varied with age. Among patients less than 12 months of age, the spinal cord ended at L2 in 73.3% of patients compared to the remainder of the cohort, who were more than 12 months of age (15.7%). A cadaveric study of Barson et al reported that by the age of 8 weeks, the spinal cord attains the adult level of L1-2 [14]. Similarly, James et al reported that the conus reached the L2 level by the age of 5 months [15]. Wilson and Prince, based on MR images, concluded that conus ends at L2-3 or above [16]. However, the study cohort included patients with tethered cord or spinal tumors. Van Schoor et al showed a difference in the level of the conus medullaris between infants (L2-3) and older children (L1) [17]. More importantly, as it related to performance of caudal epidural anesthesia, in our study cohort, the DS tapered at the level of the S2 vertebrae in the majority of the patients. In the remaining patients, the DS tapered at the S1 vertebral level or rarely at L5. A higher level of termination (above S2) was more common in patients greater than 24 months of age.
Specific limitations of this study must be recognized. All imaging was performed in the supine position, which may compress the skin and subcutaneous tissues, thereby affecting the measurements when considering the skin-to-DS measurements. The majority of neuraxial blocks in children are performed in the lateral or modified Sims position, which may affect the actual distances. Using MRI in 30 adult volunteers, Ranger et al observed the anterior movement of the spinal cord with a change in posture (from the supine to the left lateral position with knees and hips flexed). However, the cranio-caudal movement was statistically insignificant [18]. The study concluded that the absence of significant cranial displacement of the conus medullaris along the cranio-caudal axis still makes the spinal cord vulnerable to injury during lumbar neuraxial blockade at the upper lumbar levels. Koo et al showed that DS moved cephalad in the lateral flexed position used for neuraxial blockade, increasing the safety margin to avoid dural puncture caudal epidural blockade in younger children [19].
Due to the large interpatient variability in measurements, we suggest that it is not feasible to develop a single formula to estimate the depth of the DS from the skin. Given this variability, individual care is required for each patient. Miscalculation of the depth may increase the risk of accidental dural puncture during performance of a caudal epidural block. We would recommend that the needle be advanced only 1 - 2 mm once the SCM has been punctured. Despite the interpatient variability noted, the literature is reassuring in that the incidence of dural puncture during placement of a caudal epidural block is low. In the entire cohort of patients, the spinal cord ended at L1 most commonly although when considering various age ranges, the spinal cord ended most commonly at L2 in patients less than 12 months of age. The DS tapered at the level of S2 vertebrae in the majority of the patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
As a retrospective study with de-identified human data, the need for individual patient informed consent was waived.
Author Contributions
All authors were directly involved, and all reviewed and approved the manuscript. Drs. Tariq Wani and Odai Khasmah accomplished the primary writing of the text. Drs. Tariq Wani and Carmen Simion helped with study design and statistical analysis. Drs. Tariq Wani and Joseph Tobias helped with the manuscript reviews and revisions. Dr. Tobias acted as mentor and senior author in the paper.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
BSA: body surface area; DS: dural sac; MRI: magnetic resonance imaging; SCM: sacrococcygeal membrane; VIF: variance inflation factor
| References | ▴Top |
- Siddiqui A. Caudal blockade in children. Tech Reg Anesth Pain Manage. 2007;11:203-207.
doi - Patel D. Epidural analgesia for children. Contin Educ Anaesth Crit Care Pain. 2006;6:63-66.
doi - Giaufre E, Dalens B, Gombert A. Epidemiology and morbidity of regional anesthesia in children: a one-year prospective survey of the French-Language Society of Pediatric Anesthesiologists. Anesth Analg. 1996;83(5):904-912.
doi pubmed - Ecoffey C, Lacroix F, Giaufre E, Orliaguet G, Courreges P, Association des Anesthesistes Reanimateurs Pediatriques d'Expression F. Epidemiology and morbidity of regional anesthesia in children: a follow-up one-year prospective survey of the French-Language Society of Paediatric Anaesthesiologists (ADARPEF). Paediatr Anaesth. 2010;20(12):1061-1069.
doi pubmed - Polaner DM, Taenzer AH, Walker BJ, Bosenberg A, Krane EJ, Suresh S, Wolf C, et al. Pediatric Regional Anesthesia Network (PRAN): a multi-institutional study of the use and incidence of complications of pediatric regional anesthesia. Anesth Analg. 2012;115(6):1353-1364.
doi pubmed - Walker BJ, Long JB, Sathyamoorthy M, Birstler J, Wolf C, Bosenberg AT, Flack SH, et al. Complications in pediatric regional anesthesia: an analysis of more than 100,000 blocks from the pediatric regional anesthesia network. Anesthesiology. 2018;129(4):721-732.
doi pubmed - Llewellyn N, Moriarty A. The national pediatric epidural audit. Paediatr Anaesth. 2007;17(6):520-533.
doi pubmed - Meyer MJ, Krane EJ, Goldschneider KR, Klein NJ. Case report: neurological complications associated with epidural analgesia in children: a report of 4 cases of ambiguous etiologies. Anesth Analg. 2012;115(6):1365-1370.
doi pubmed - Imbelloni LE, Fornasari M, Fialho JC. Combined spinal epidural anesthesia during colon surgery in a high-risk patient: case report. Rev Bras Anestesiol. 2009;59(6):741-745.
doi - Kasai T, Yaegashi K, Hirose M, Tanaka Y. Spinal cord injury in a child caused by an accidental dural puncture with a single-shot thoracic epidural needle. Anesth Analg. 2003;96(1):65-67.
doi - Adewale L, Dearlove O, Wilson B, Hindle K, Robinson DN. The caudal canal in children: a study using magnetic resonance imaging. Paediatr Anaesth. 2000;10(2):137-141.
doi pubmed - Blanchais A, Le Goff B, Guillot P, Berthelot JM, Glemarec J, Maugars Y. Feasibility and safety of ultrasound-guided caudal epidural glucocorticoid injections. Joint Bone Spine. 2010;77(5):440-444.
doi pubmed - Lee RA, van Zundert AA, Botha CP, Lataster LM, van Zundert TC, van der Ham WG, Wieringa PA. The anatomy of the thoracic spinal canal in different postures: a magnetic resonance imaging investigation. Reg Anesth Pain Med. 2010;35(4):364-369.
doi pubmed - Barson AJ. The vertebral level of termination of the spinal cord during normal and abnormal development. J Anat. 1970;106(Pt 3):489-497.
- James CCM, Lassman LP. Spinal dysraphism. In: Spina bifida occulta. London: Butterworth, 1972; p. 20-21.
- Wilson DA, Prince JR. John Caffey award. MR imaging determination of the location of the normal conus medullaris throughout childhood. AJR Am J Roentgenol. 1989;152(5):1029-1032.
doi pubmed - Van Schoor AN, Bosman MC, Bosenberg AT. Descriptive study of the differences in the level of the conus medullaris in four different age groups. Clin Anat. 2015;28(5):638-644.
doi pubmed - Ranger MR, Irwin GJ, Bunbury KM, Peutrell JM. Changing body position alters the location of the spinal cord within the vertebral canal: a magnetic resonance imaging study. Br J Anaesth. 2008;101(6):804-809.
doi pubmed - Koo BN, Hong JY, Kim JE, Kil HK. The effect of flexion on the level of termination of the dural sac in paediatric patients. Anaesthesia. 2009;64(10):1072-1076.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
International Journal of Clinical Pediatrics is published by Elmer Press Inc.
